Somayeh Sadat Shariatmaghani MD1, Reza Jafarzadeh Esfehani PHD2, Amir Hossain RashidpourMSc3, Sedigheh Shariat Moghani MSc4*
1Fellowship, Department of internal medicine, Mashhad University of Medical Sciences, Mashhad, Iran
2Assistant Professor, Department of Basic Genetics Sciences, Mashhad University of Medical Sciences, Mashhad, Iran
3MSc Student in HTA, Department of Health, Shahid Sadoughi Univercity of Medical sciences, Yazd, Iran
4Faculty of Member, Department of Midwifery, School of Nursing and Midwifery, Mashhad University of Medical Sciences, Mashhad, Iran
*Corresponding Author: Sedigheh Shariat Moghani MSc, Faculty of Member, Department of Midwifery, School of Nursing and Midwifery, Mashhad University of Medical Sciences, Mashhad, Iran.
Abstract
Background & aim: The recent coronavirus disease 2019 (COVID-19) is a global health issue affecting almost every population. The effect of COVID-19 on pregnancy outcomes and its management during pregnancy is still unclear. Some studies have reported the use of corticosteroids during different trimesters of pregnancy and their relationship to adverse pregnancy outcomes; however, their potential benefits and side effects are not widely studied in pregnant women with COVID-19. Therefore, the present review was carried out to systematically evaluate the available literature about the effects of corticosteroids on adverse pregnancy outcomes in pregnant women with SARS-CoV-2 infection.
Methods: This systematic review were searched on international databases (Embase, Scopus, Pubmed, Science Direct, Web of Science)and included case report studies, to find out the articles published from 1 February 2020 to 30 August 2022 reporting corticosteroid treatment during pregnancy in COVID-19 positive patients and was performed by the PRISMA checklist.
Keywords were selected based on Mesh (“Pregnancy”, “corticosteroid”, “Delivery”, “Infant”, “Fetus”, “Outcome”, “Complication”, “Abortion”, “Premature”, “Fetal Death”, “COVID19” “Coronavirus Infection”). The full texts of articles were reviewed by two independent reviewers and the relevant data was extracted.
Results: Although the available data is obtained only from the small number of pregnant women receiving different typesof corticosteroids with different dosage and duration, however, fetal mortality was not reported. According to the results, there is still a lack of large clinical trials on the use of corticosteroids in pregnant patients with COVID-19 and their effectson pregnancy outcomes.
Conclusion: Although short term corticosteroids may reduce inflammation and prevent the development of respiratory distress; however, clinical confirmations in controlled trials are needed. Corticosteroid administration shouldbe constantly discussed by a multidisciplinary team with specific considerations for pregnant women in different trimesters.
Keywords: Coronavirus, Pregnant Women, Corticosteroid, systematic review
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS- COV-2), rapidly became a respiratory syndrome coronavirus (MERS-CoV) or severe acute respiratory syndrome coronavirus (SARS-CoV), the COVID-19 had many therapeutic challenges during the first months ofthe pandemic as there was not enough clinical evidence regarding the pathogenesis and effective treatments of the disease [2]. While there was not any definite treatment for COVID-19, some clinical trials evaluated drug regimens that were effective on previous coronavirus infections and some used treatments interfering with the disease pathogenic mechanisms in the pregnant women body [3]. There are still many therapeutic challenges after more than two year of the first confirmed COVID-19 patients. One of the main therapeutic challenges is the use of corticosteroids in pregnancy and COVID-19 [4]. There are controversial results regarding the indication to begin, the best type and the optimal dose of corticosteroids for managing COVID-19 [5]. Pregnant women experience to accommodate and tolerate the growing fetus, immune system changes. These changes also increase their susceptibility to viral infections such as SARS-COV-2. COVID- 19 in pregnancy and increasesthe likelihood of hospital admission and intensive care compared to non-pregnant women [4].
A most recent systematic review of the clinical trials and observational studies considering corticosteroids in therapeutic regimens for COVID-19 demonstrated that although the corticosteroids are helpful in reducing short term mortality and the need for mechanical ventilation; however, the optimal dose, time and duration of therapy as well as the risk of developing secondary infections and delayed viral clearance is still a concern [5]. Another challenge during the COVID-19 outbreak is the infection of pregnant women by SARS-CoV-2. The short and long-term effects of SARS- CoV-2 infection during pregnancy and its effect on pregnancy outcomes are still unknown [6]. Moreover, the effect of corticosteroids during different trimesters of pregnancy and their relationship with adverse pregnancy outcomes is not widely studied. Magala Ssekandi et al (2021) show that early administration of low-dose corticosteroids to patients with acute respiratory distress syndrome can reduce all-cause mortality among such patients. Also, during pregnancy, prolonged use of corticosteroids that readily cross the placenta like dexamethasone can negatively impact both the mother and foetus. Pregnant women in this trial received either oral prednisolone or intravenous hydrocortisone [3]. In a review study of COVID-19 therapeutics in pregnancy (2022) report that there are many important knowledge gaps about the safety and efficacy of key therapeutics used for COVID-19 in pregnancy [4].
In another study (2021) show that increased coagulation and risk of thromboembolic phenomena are common during pregnancy; they are further enhanced when associated with a thrombogenic pathology such as in COVID-19. The treatment of pregnant women with COVID-19 brings important peculiarities that should be considered in order to make better decisions for preserving the health of the mother and fetus [5]. According to the literature, So far, there are no published articles on corticosteroid use among pregnant women with covid-19 disease. Therefore, the present review study was performed aimed to systematically evaluate the available literature on the effect of corticosteroids on adverse pregnancy outcomes in pregnant women with SARS-CoV-2 infection.
Materials and Methods
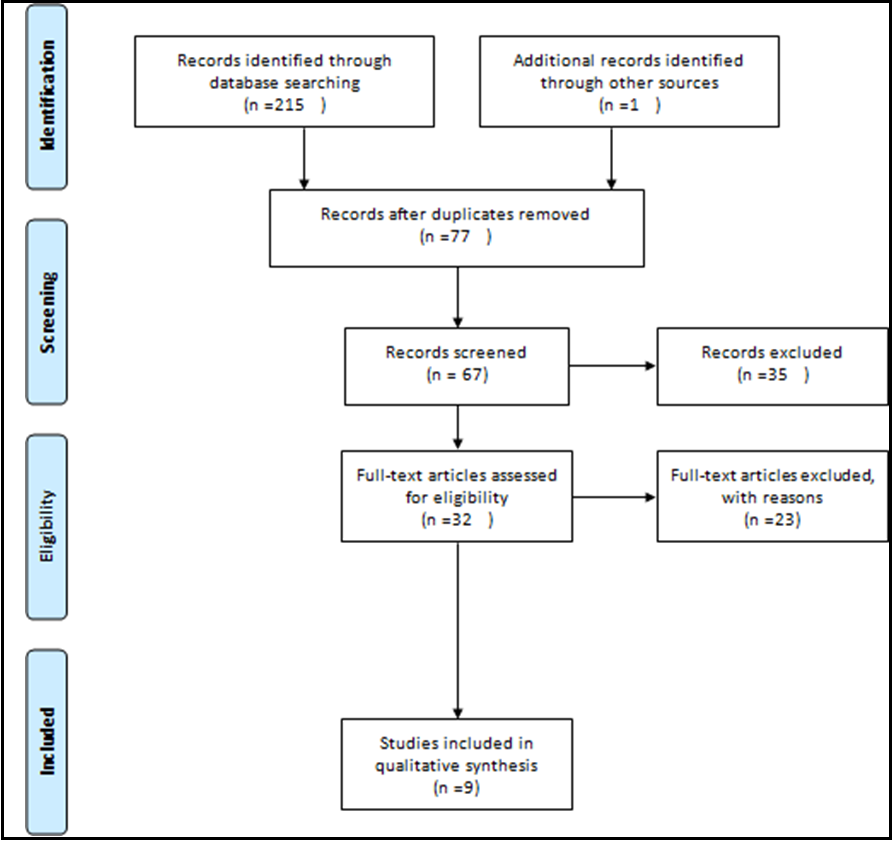
This systematic review study was conducted without time limitation until August 2022. This study was performed according to the protocol of systematic review and meta-analysis articles (PRISMA) (Figure 1).
Figure 1: PRISMA flow diagram
This systematic review study was approved by the Ethic Committee of Mashhad University of Medical Sciences with the ethical code of IR.MUMS.REC.1399.209. The inclusion criteria were: cross- sectional, clinical trial, case report and cohort studies that examined Corticosteroid use in pregnant women with Coronavirus. Only the studies which have started any types of corticosteroids regardless of the administration route or dosage were included. Studies without full-text access were not included. Among these studies, only those indicating the pregnancy outcomes were selected for further evaluation. Exclusion criteria were: lack of relevance to the subject, insufficient data, review articles, and letter to editors. Only articles in Englishwere included. After considering the inclusion and exclusion criteria, the final records were then evaluated by case Series or case report Critical Appraisal Tool as the conclusive studies where all case reports or case series [7].
To identify the related studies, two independent researchers searched in national and international databases, including: (EMBASE, Scopus, Pubmed, Science Direct, Web of Science) as well as Google Scholar search engine. The references used in the articles were also reviewed to access other related studies.
Keywords used in the search strategy were:
“coronavirus”, "coronavirus pneumonia", "COVID-19", "2019 novel coronavirus infection", “2019-nCoV", and “corticosteroid*”, “steroid*”, “methylprednisolone”, “hydrocortisone”, “prednisolone”, “prednisone”, “dexamethasone”, “cortisol”, “glucocorticoid*”, and "pregnant”, “pregnancy”.
Unique search strategies have been developed using the Medical librarian specializing in systematic review search using MESH expressions and open expressions by PRESS standards. After finalizing the MEDLINE strategy, the results were compared to a search of other databases.
For studies' selection, all relevant articles were first collected, and after the search, a list of abstracts was prepared. The full text of the articles was provided to the researchers after hiding the details of the articles, including the name of the journal and author. Each article was studied by two researchers independently, andif the article was rejected, the reason for rejection was mentioned. If there was disagreement between the two researchers, the article was judged by a third researcher.
The quality assessment of this study was done by two authors (MT-S SH) individually using the JBI Critical Appraisal Checklist for Case Reports. The full text was prepared for review if the title or abstract was relevant to the present study. General information (first author, country, and year of publication) and study information (number of participants and mean age) were evaluated.
Results
A Total of 216 studies were retrieved according to the search strategy, and after removing the duplicated references, 77 studies remained. Among these studies, one study was excluded because it evaluated pregnant women with previous SARS infection [8]. After removing non-English language articles as well as the irrelevant article types, 9 articles remained that were eligible for further evaluation [6,9-16]. These studies were case reports or case series on pregnant women affected with SARS-CoV-2 infection (Table 1).
Table 1. The clinical case reports/case series addressed the administration of corticosteroids during pregnancy.
|
Author |
Mother’s age (year)/underlying diseases |
Mother disease severity |
(Pregnancy week) Of using corticosteroids |
Corticostero id and dose |
Other drugs |
Maternal outcome |
Gestational age (weeks) |
Main fetus outcome |
|
Browne et al. [9] |
33/ asthma and migraine |
Mild |
23* |
Prednisone |
Azithromycin, acetaminophen |
Pregnancy continued |
23 |
preterm labor and extremely low birth weight resolved |
|
Hong et al. [10] |
36/hypothyroidism, Morbid obesity, hyperlipidemia |
Severe |
23* |
Oral prednisone 80 mg twicedaily for a total of 7 days |
Hydroxychloro quine for 5 days |
Pregnancy continued |
23 |
Without preterm delivery |
|
Fontanella et al. [6] |
38 |
Moderate |
31* |
- |
Hydroxychloro quine, low molecular weight heparin |
Pregnancy continued |
33 |
Well established. benefit on neonatal morbidity and mortality |
|
Kolkova et al. [11] |
27/ type 1diabetes, hypothyroidism |
Moderate |
29* |
- |
Tinzaparin |
nosocomial superinfection |
32 |
Normal |
|
Soleimani et al. [12] |
30/obesity |
Severe |
21* |
Methylpredn isolone (500 mg bid) for two days, followed by intravenous hydrocortisone in divided doses |
Lopinavir/riton avir and azithromycin |
better clinical outcome |
21 |
normalfetal growth |
|
Du Fosse et al. [13] |
30/subclinical hypothyroidism |
Severe |
23* |
Oral prednisolone (60 mg) daily |
- |
- |
28 |
Preterm morbidities^ |
|
Jacobson et al. [14] |
42 |
Severe |
26* |
dexamethaso ne 20 mg IV for 5 days followed by 10 mg IV for 5 days |
Remdesivir, convalescent plasma, azithromycin ceftriaxone |
- |
29 |
adrenal insufficiency |
|
Alsayyed et al. [15] |
34 |
Severe |
26* |
50 mg of methylpredni solone four times daily for 25 days |
Ceftriaxone, azithromycin |
- |
39 |
Normal |
|
Gupta et al. [16] |
20 |
mild |
22* |
intravenous dexamethasone (at 6 mg/day) |
vancomycin and piperacillin/ tazobactam |
clinical improvement, Pregnancy continued |
22 |
favorable outcome with preservation of pregnancy |
*corticosteroid used for lung maturation and the exact dose or administration root or duration were not clear
Most of the pregnant women in these studies had moderate to severe COVID-19 [6,10-15]. In five studies out of 9 studies, COVID-19 pregnant women were successfully managed, but they were not followed up for delivery outcomes. Four out of 9 studies were performed on women with unremarkable previous medical history [6,14-16]. Corticosteroid therapy started prior to 30 weeks of gestation except in one study [6]. All the studies used corticosteroids for lung maturation, and no maternal mortality during pregnancy was reported [6,9-16]. Among the enrolled studies, 2 studies used intravenous dexamethasone with considerably different doses [14,16]. While Jacobson et al. used 20 mg of intravenous dexamethasone for 5 days and reduced the corticosteroid to 10 mg for the next five days, Gupta et al. used a daily dose of 6 mg intravenous dexamethasone [14,16]. While the pregnancy outcome in the study of Gupta et al. is not reported, Jacobson et al. reportedthat the patients developed a preterm baby with adrenal gland insufficiency [14,16]. Soleimani et al. used a daily dose of 1000 mg methylprednisolone for 2 days, and Alsayyed et al. used 200 mg daily methylprednisolone for 25 days [12,15]. While the pregnancy outcome in the study of soleimani et al. is not reported yet, but Alsayyed et al. reported that the patient had standard term delivery [12,15]. Du Fosse et al., in their study, demonstrated that 60 mg of oral prednisolone daily ended up in delivery at 28 weeks with preterm comorbidities [13]. Both Browne et al. and Hong et al. studies used prednisone, but none of them reported the pregnancy outcome; and only the Hong et al. study demonstrated the exact corticosteroid dose (160 mg daily for 7 days) [9,10]. When considering the case series or case report Critical Appraisal Tool for the 9 studies included in the present study, only 4 studies had sufficient quality and demonstrated their patients’ outcomes [11,13- 15]. However, none of these studies used the same type or doses of corticosteroids and therefore, it is not possibleto make a conclusion regarding the effect of corticosteroids on pregnancy outcomes [11,13-15].
Discussion
The present review summarized the clinical studies on the use of corticosteroids in pregnant women infected with SARS-CoV-2. Despite recent achievements in understanding the clinical and pathophysiological mechanisms of SARS-CoV-2 infection in humans, choosing the most effective treatment still needs to be discussed. Corticosteroids are one of these treatments, which is considered alone or in combination with other drugs during the COVID-19 course. These drugs are considered a double edge sword according to their specific effect on controlling the inflammatory responses and affecting the immune system potency [17,18]. Early in the pandemic, the guidelines did not recommend the use of corticosteroids for COVID-19 patients in most countries but later become an interesting topic for researchers [19]. The clinical use of corticosteroids has been questioned and addressed in recent meta- analysis studies [4,20,21]. The most recent meta-analysis suggested the use of corticosteroids in severe COVID-19 and prohibited it in patients not requiring oxygen therapy [4]. On the other hand, a meta- analysis of 21350 patients demonstrated that the overall mortality rate is higher among those COVI-19 patients receiving corticosteroids compared to those who did not use corticosteroids [20]. While the efficacy of corticosteroids in severe COVID-19 patients is becoming clearer, the dosage and duration of corticosteroid therapy are still controversial [21].
Studying the effect of corticosteroid therapy on pregnant women has been left neglected. The effect of SARS- Cov-2 infection on pregnant women has been addressed in various clinical trials, but the controversial result is also present for the possible relation between COVID-19 and adverse pregnancy outcomes. While some meta- analysis addressed the increased rate of preterm labor during the COVID-19 pandemic, some others demonstrated that the pandemic did not have a specific effect on preterm labor and adverse pregnancy outcomes [22-24]. The literature review in the present study showed that most of the clinical trials and even cohort studies excluded pregnant women from their research, and therefore the only available data on this population is based on case reports. Corticosteroids have been administered to pregnant people for a long time ago. These drugs are among the common treatments administered between 23 and 34 weeks of gestation to promote lung maturation in mothers who are susceptible to premature birth [25]. Administration of corticosteroids in pregnant women with increased risk of preterm delivery has been reported to be beneficial in reducing neonatal respiratory complications [26]. Corticosteroids are suggested to be used in 2 courses of 2 doses with 24 hours intervals in order to prevent possible adverse neonatal outcomes.
Our systematic search of the available literature demonstrated that most of the available data regarding the effect of corticosteroid therapy in pregnant women with COVID-19 is obtained from case reports and many clinical studies excluded the pregnant population. In contrast to a large number of clinical trials excluding pregnant women, the RECOVERY trial recommended prednisolone uses in COVID-19 (intravenous hydrocortisone 80mg twice daily or 40mg of oral prednisolone). However, this result was obtained based on 6 pregnant patients involved in the trial [27]. Based on the result of this study, consistent with the results of previous experiments on other viral infections during pregnancies, specific use of corticosteroids during pregnancy has been suggested by some authors, and some questions have been raised. While the effect of corticosteroid therapy in pregnant women diminishes over time, the perfect timing for corticosteroid therapyshould be considered in every patient. Previous experiences during previous SARS-CoV infections indicated delayed viral clearance and worse outcomes for those receiving corticosteroids. The common corticosteroid dosing for most obstetric purposes is 12mg of intramuscular injection betamethasone 24h apart, which is equivalent to 320mg of hydrocortisone used in MERS treatment [28]. On the other hand, regarding the recommendation of using corticosteroids for women at risk of preterm delivery between 23 and 36 weeks, McIntosh et al. recommended that pregnant women with COVID-19 should not receive corticosteroids after the 32nd weeks of gestation and administration of corticosteroids before this time should be discussed by a multidisciplinary team [29]. Moreover, the most recent commentary by Saad et al. suggested the use of corticosteroid therapy in pregnant women requiring oxygen therapy or ventilation support. They also suggested 4 dose courses of dexamethasone over 48 hours when long fetal maturity is required in a pregnant mother who requires oxygen therapy or mechanical ventilation. Dexamethasone should be replaced by methylprednisolone in a 10-day course [30].
Regarding the limited evidence on the evaluation of the effect of corticosteroids on pregnant women, some authors used decision model analysis. Parker et al. published a decision model analysis about antenatal corticosteroid use in high-risk women for preterm labor. They demonstrated that corticosteroid use prior to 30 weeks of gestation in COVID-19 pregnant women admitted to ICU and 32 weeks for those who are only hospitalized is associated with improved outcomes for mother and infant [31]. Another decision analysis modelling study evaluating the effect of antenatal corticosteroids on pregnant women with PPROM conductedby Zhou et al. demonstrated that among 10000 pregnant women with COVID- 19 and PPROM between 24 to 32 weeks of gestation, administration of antenatal corticosteroids resulted in fewer RDS, intraventricular hemorrhage and infant mortality. Moreover, they demonstrated that infants with lower gestational age (less than 31 weeks) are more likely to benefit from antenatal corticosteroids [28].
Our systematic literature search showed that only 9 articles reported corticosteroid use in pregnant women with COVID-19. Based on these studies, the antenatal use of corticosteroids for lung maturation has been considered for every patient. Even though most of the reported patients had moderate to severe disease, noneof these studies reported perinatal death. There was a considerable variation regarding the dosage, administration route and duration of different corticosteroids, which makes the comparison of the studies impossible [6,9-16]. However, the favorable effect of corticosteroids on fewer ARDS and mortality rate is early administration, as almost all of the patients had received corticosteroids prior to the 30 weeks of gestation. Another challenging issue that is evident in these studies is the other treatment regimens used alongside corticosteroids. It is unclear whether the use of corticosteroids in combination with these drugs prevented the possible mortality.
The number of sample size and quality evaluation of included articles by 3 authors was one of the strengths of the study. Inadequate information about the end of pregnancy and appropriate treatment and limitations in the selection of articles are among the weak points of the study.
In this study, there was evidence of the need for rapid treatment of pregnant women infected with Covid-19.
Early treatment reduces negative maternal and neonatal outcomes in the future.
Further studies to collect more robust data are needed to further confirm or prove these points Identification of effective treatment strategies for Preventing adverse outcomes in pregnant people with Covid-19 Recommended.
Conclusion
The present systematic review demonstrated a lack of large clinical trials on corticosteroid use in pregnantwomen with COVID-19 and their effects on pregnancy outcomes. Although some studies have reported that shortterm corticosteroids may reduce inflammation and prevent the development of ARDS; however, clinical confirmations in controlled trials and especially in the pregnant population are needed. Although the available data is obtained only from the small number of pregnant women receiving different types of corticosteroids with different dosage and duration, however, fetal mortality was not reported. Since there is inadequate data regarding the use of corticosteroids in pregnant women with COVID-19 infection, it seems that before the publication of large clinicaltrials, corticosteroid administration should always be discussed by a multidisciplinary team with specific considerations on pregnant women in different trimesters.
Acknowledgements
The present study has been funded by Mashhad University of Medical Sciences Research committee. The authorswould like to acknowledge Mashhad University of medical sciences research committee for supporting the present research.
Conflicts Of Interest: Authors declared no conflicts of interest.
References
- Park M, Cook AR, Lim JT, Sun Y, Dickens BL (2020) A systematic review of COVID-19 epidemiology based on current evidence. Journal of clinical medicine. 9(4): 967.
- Yuki K, Fujiogi M, Koutsogiannaki S (2020) COVID-19 pathophysiology: A review. Clinical immunology. 215: 108427.
- Ssekandi AM, Sserwanja Q, Olal E, Kawuki J, Adam MB (2021) Corticosteroids use in pregnant women with COVID-19: recommendations from available evidence. Journal of multidisciplinary healthcare. 14: 659-663.
- Jorgensen SC, Tabbara N, Burry L (2022) A review of COVID-19 therapeutics in pregnancy and lactation. Obstetric Medicine. 15(4): 225-232.
- Rios SS, Resende CN, Peixoto AB, Júnior EA (2021) Treatment of COVID-19 disease in pregnancy and breastfeeding. Сеченовский вестник. 12(2): 44-54.
- Fontanella F, Hannes S, Keating N, Martyn F, Browne I, et al. (2020) COVID-19 infection during the third trimester of pregnancy: Current clinical dilemmas. European journal of obstetrics, gynecology and reproductive biology. 251: 268-271.
- Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, et al. (2017) Systematic reviews of etiology and risk. 17(5).
- Shek CC, Ng PC, Fung GPG, Cheng FWT, Chan PKS, et al. (2003) Infants Born to Mothers With Severe Acute Respiratory Syndrome. 112(4): e254.
- Browne PC, Linfert JB, Perez-Jorge E (2020) Successful Treatment of Preterm Labor in Association with Acute COVID- 19Infection. American journal of perinatology. 37(08): 866-868.
- Hong L, Smith N, Keerthy M, Lee-Griffith M, Garcia R, et al. (2020) Severe COVID-19 infection in pregnancy requiring intubation without preterm delivery: a case report. Case reports in women's health. 27: e00217.
- Kolkova Z, Bjurström MF, Länsberg J-K, Svedas E, Hamer MA, et al. (2020) Obstetric and intensive-care strategies in a high-risk pregnancy with critical respiratory failure due to COVID-19: A case report. Case reports in women's health. 27: e00240.
- Soleimani Z, Soleimani A (2020) ADRS due to COVID-19 in midterm pregnancy: successful management with plasma transfusion and corticosteroids. The Journal of Maternal-Fetal & Neonatal Medicine. 13(7): 1-4.
- du Fossé NA, Bronsgeest K, Arbous MS, Zlei M, Myeni SK, et al. (2021) Detailed immune monitoring of a pregnant woman with critical Covid-19. Journal of reproductive immunology. 143: 103243.
- Jacobson J, Antony K, Beninati M, Alward W, Hoppe KK (2021) Use of dexamethasone, remdesivir, convalescent plasma and prone positioning in the treatment of severe COVID-19 infection in pregnancy: A case report. Case reports in women's health. 29: e00273.
- Alsayyed F, Hastings V, Lederman S (2020) Expectant Management of a Critically Ill Pregnant Patient with COVID-19 with Good Maternal and Neonatal Outcomes. Case reports in obstetrics and gynecology. 25(2020): 6. 8891787.
- Gupta S, Kaushik A, Kest H, Charles AK, De Bruin W, Colletti M, et al. (2020) Severe Enteritis as the Sole Manifestation of Novel Coronavirus Disease 2019 (COVID-19) in Adolescent Patients. Case reports in infectious diseases. 23(2020): 8823622.
- Mishra GP, Mulani J (2021) Corticosteroids for COVID-19: the search for an optimum duration of therapy. The Lancet Respiratory medicine. 9(1): e8.
- Ellsworth GB, Glesby MJ, Gulick RMJJIM. (2021) The uncertain role of corticosteroids in the treatment of COVID-19. JAMA Internal Medicine. 181(1): 139-140.
- Dagens A, Sigfrid L, Cai E, Lipworth S, Cheng V, et al. (2020) Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: rapid review. BMJ (Clinical researched). 369: m1936.
- Cano EJ, Fonseca Fuentes X, Corsini Campioli C, O'Horo JC, Abu Saleh O, et al. (2020) Impact of Corticosteroids in Coronavirus Disease 2019 Outcomes: Systematic Review and Meta-analysis. Chest. 159(3): 1019-1040.
- Kheyami ZA, Alharthi SS, Alanazi NS, Awad MA, Aldosari AM, et al. (2021) Systemic Steroids and Mortality Rate in Severe COVID-19 Infection: Systematic Review and Meta-analysis. Annals of Medical and Health Sciences Research. 11: 1208-1211.
- Dubey P, Reddy SY, Manuel S, Dwivedi AK (2020) Maternal and neonatal characteristics and outcomes among COVID-19 infected women: An updated systematic review and meta-analysis. European Journal of Obstetrics & Gynecology and Reproductive Biology. 252: 490-501.
- Matar R, Alrahmani L, Monzer N, Debiane LG, Berbari E, et al. (2020) Clinical Presentation and Outcomes of Pregnant Women with COVID-19: A Systematic Review and Meta-Analysis. Clinical Infectious Diseases. 72(3): 521-33.
- Gao YJ, Ye L, Zhang JS, Yin YX, Liu M, et al. (2020) Clinical features and outcomes of pregnant women with COVID-19: a systematic review and meta-analysis. BMC Infectious Diseases. 20(1): 1-11.
- Kakoulidis I, Ilias I, Koukkou E (2020) SARS-CoV-2 infection and glucose homeostasis in pregnancy. What about antenatal corticosteroids. Diabetes & metabolic syndrome. 14(4): 519-520.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, Tita AT, Reddy UM, et al. (2016) Antenatal betamethasone for women at risk for late preterm delivery. New England Journal of Medicine. 374(14): 1311-1320.
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. (2021) Dexamethasone in hospitalized patients with Covid-19. New England Journal of Medicine. 384(8): 693-704.
- Zhou CG, Packer CH, Hersh AR, Caughey AB (2022) Antenatal corticosteroids for pregnant women with COVID-19 infection and preterm prelabor rupture of membranes: a decision analysis. The Journal of Maternal-Fetal & Neonatal Medicine. 35(9): 1643- 1651.
- McIntosh JJ (2020) Corticosteroid Guidance for Pregnancy during COVID-19 Pandemic. American Journal of Perinatology. 37(8): 809-812.
- Saad AF, Chappell L, Saade GR, Pacheco LD (2020) Corticosteroids in the management of pregnant patients with coronavirus disease (COVID-19). Obstetrics & Gynecology. 136(4): 823-826.
- Packer CH, Zhou CG, Hersh AR, Allen AJ, Hermesch AC, et al. (2020) Antenatal Corticosteroids for Pregnant Women at High Risk of Preterm Delivery with COVID-19 Infection: A Decision Analysis. American journal of perinatology. 37(10): 1015-21.