Wolfram Schummer1,2*
1HELIOS Spital Ueberlingen, 88662 Ueberlingen, Germany
2Friedrich Schiller University Jena, 07747 Jena, Germany
*Corresponding Author: Wolfram Schummer, HELIOS Spital Ueberlingen, 88662 Ueberlingen, Germany, Friedrich Schiller University Jena, 07747 Jena, Germany.
Abstract
Introduction: Optimal tip location is paramount for central venous catheters (CVC) to function correctly.
One technique to verify CVC position is the ECG method. Nowadays, the ECG method is applied using the maximum P-wave amplitude (P- max).
The hypothesis is that a precise method in assessing CVC position can provide the same results for CVC tip positions regardless of their respective insertion sites.
Methods: Only critically ill patients with multiple organ dysfunction were eligible for the study. Another condition was a prerequisite for reliable illustration of the results, i.e., at least two central venous lines had to be in place. All catheters were placed using the ECG method with the CVC tip at P-max. A chest X-ray was performed within 24 hours of line insertion in all patients to assess the CVC positions.
Results: Between January 2018 and December 2020, 51 critically ill patients with more than one CVC concurrently in place were deemed eligible and were included in the study. The distance between the inserted CVC tips was measured using the picture archiving and communication system (PACS). Across 51 patients, the mean difference between the two central lines inserted was 0.31 cm. The median was 0.00 cm, indicating that more than half of the sample exhibited no difference in positioning.
Conclusion: The ECG method of placing the CVC tip at P-max is a stable and reliable bedside method for positioning CVCs as the results for double CVCs impressively underline, and it already works during the access procedure. However, the results demand confirmation in further studies.
Key Words
Central venous catheterization;
ECG method;
echocardiography; transoesophageal;
chest x-ray;
vena cava, superior
Key Points Question
Can the ECG method (at P-max) provide the same results for the position of CVC tips regardless of their insertion site?
Results
Across 51 patients, the mean difference between two central lines concurrently in place was 0.31 cm – the median was 0.00 cm.
Meaning
The ECG method for placing CVC tips at P-max is a reliable, precise bedside method for positioning CVC tips and already works during the access procedure.
Introduction
Central venous catheters (CVC) are essential in managing critically ill patients by measuring hemodynamic variables that cannot be measured accurately by non-invasive means and by allowing delivery of medications and nutritional support that cannot be given safely through peripheral venous catheters. [1] Unfortunately, these catheters are not without the potential for harm. [1] The insertion procedure, in particular, carries the risk of severe mechanical complications, [2] though ultrasound imaging may dramatically reduce this risk. [2]
For the catheter to function correctly, tip location is of utmost importance. [3,4] Inserting the tip too far into the right atrium raises severe arrhythmias or even pericardial tamponade risks. [4,5] Inserting it too shallowly – in the innominate vein or the upper third of the superior vena cava – poses the risk of intimal damage and venous thrombosis, fibrin sleeve formation, and persistent withdrawal occlusion. [4,6,7,8,9] Even with correct initial positioning, these catheters are prone to tip migration. [10] However, the risk of erosion and even perforation of the vein wall should not be ignored in light of their intensity. [10,11]
The ECG method of siting CVC tips has developed markedly over recent decades. [12-14] At present, the ECG method with its new interpretation – CVC tip at the maximum P-wave amplitude (P-max) – is a stable and reliable bedside method for positioning CVC tips precisely at the transition of the right atrium (RA) and superior vena cava (SVC) in patients in sinus rhythm. [13,14] This method directly enables the operator to assess the correct CVC position during insertion. [13,15] This study investigates the hypothesis that a technique that is a precise approach to assessing CVC position can provide the same results for CVC tips regardless of respective insertion sites.
Methods
This prospective single-center study was performed in the Department of Anaesthesiology and Intensive Care Medicine between January 2018 and December 2020. The University's Institutional Review Board (IRB) registered and approved the study protocol (1518-03/05). The IRB waived the requirement for written informed consent.
Only critically ill patients on vasopressor support and with multiple organ dysfunction were eligible for the study. Another condition was a prerequisite for reliable illustration of the results, i.e., at least two central venous lines had to be in place. All catheters were placed using the ECG method (Einthoven lead II) with the CVC tip at the maximum P-wave amplitude (P-max). In total, 51 patients in sinus rhythm were enrolled in the study. Sixteen (31.4 %) were female, and 35 (68.6 %) were male. Of the 102 CVCs placed, 31 (30 %) were dialysis catheters for continuous renal replacement therapy (CRRT). The baseline characteristics of the study population are summarised in Table 1.
The decision about the CVC insertion site was consistently individualized and taken on clinical grounds. This individualization and the frequently variable time points at which CVCs were placed may explain the heterogenous distribution of access sites and the composition of access groups, e.g., CVCs via the right internal jugular (RIJV) and left subclavian (LSV) routes.
During each central venous access procedure, the correct position and depth of insertion were estimated by the ECG method using the Certodyn® universal adaptor (Fa. B. Braun Melsungen AG, Germany). This switch enables the operator to change from a surface ECG (using Einthoven lead II) to an intravascular ECG. Here, the guide wire was used as a unipolar electrode. A black marking on its proximal end indicates the point at which the tip is just level with the port of the distal catheter lumen. A sterile connection cable was clamped to the guide wire at the marked position to connect the wire to the adaptor. The measurement was performed while advancing the catheter, with the Seldinger wire serving as the intraluminal ECG lead. The line tip was placed at the point of maximum P-wave amplitude for each measurement, and the insertion depth was measured.
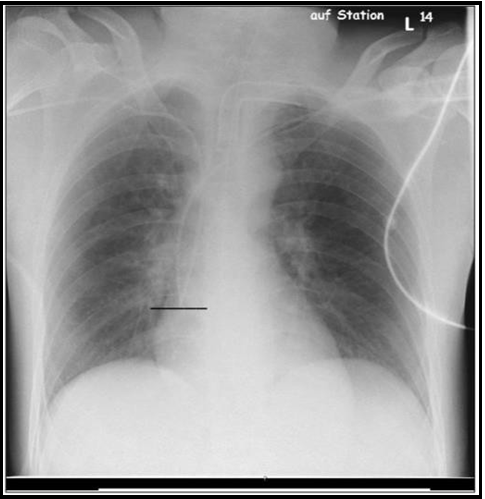
In all patients, a chest X-ray (CXR, anterior–posterior, patient recumbent) was performed within 24 hours of line insertion to assess the CVC positions. According to our hypothesis, the tips of two CVCs were expected to be at one level on CXR (Figure 1). However, due to limitations in the insertion length – 21 cm - of the CVCs applied, differences of up to two centimeters were deemed satisfactory.
Figure 1: Two central lines via the right and left subclavian vein leveling at P-max (see line )
Statistics
All data were analyzed using R Core Team (2021, R Foundation for Statistical Computing, Vienna, Austria). Patient characteristics were considered via descriptive statistics. The reliability of the ECG method was assessed via the distance between the CVC tips. Normal distribution was ruled out by the Shapiro–Wilk test with p < 0.05, so the Wilcoxon test was used to analyze tip collocation and for measurements in each insertion site group. A p-value < 0.05 was considered statistically significant.
Results
Between January 2018 and December 2020, 51 critically ill patients with more than one CVC concurrently in place were deemed eligible and included in the study. Septic shock with multiple organ failure was the most frequent reason for inserting the central lines. Almost one-third of the 102 CVCs inserted (n = 31) were hemodialysis catheters for CRRT in this vulnerable group. The clinical course of these patients often began with a critical illness and several organ dysfunctions requiring resuscitation with fluids and vasopressors. At this stage, a central line was inserted. After 48 to 72 hours, a hemodialysis catheter was placed, and CRRT started in patients whose kidney function declined to oliguria rather than showing signs of recovery.
These individual time courses led to complex CVC arrangements that we classified into several groups according to access sites and sides, e.g., CVCs were inserted right- and left-sided into the internal jugular and subclavian veins, respectively.
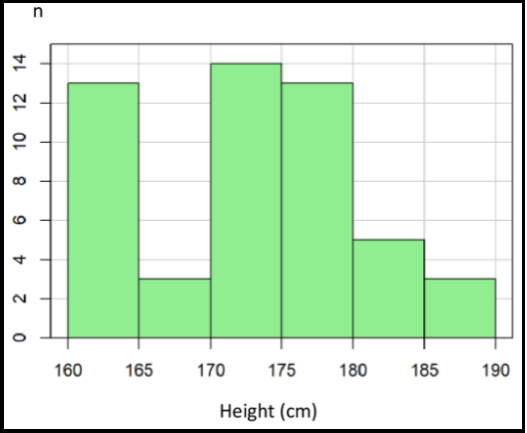
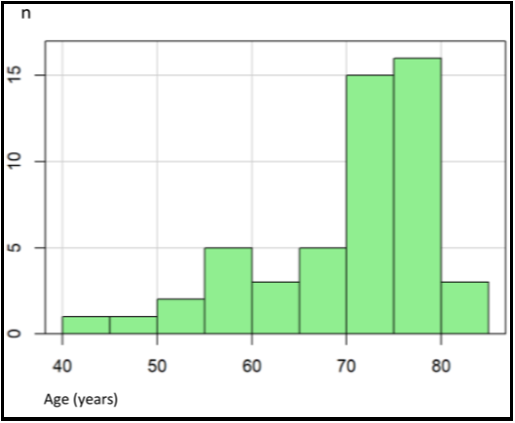
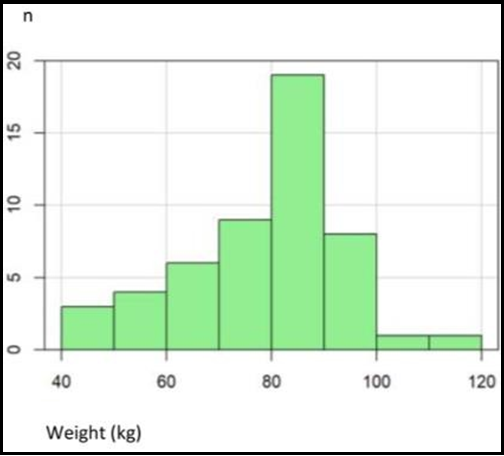
Patient characteristics are illustrated in Table 1. The patients were between 44 and 84 years, with a mean age of 70.5 (see Appendix Figure 1). This population's body height was between 160 and 186 cm, with a mean of 173 cm (Figure 2). The mean body weight was 80 kg, with a range of 46–115 kg (see Appendix Figure 2). Body mass index was normally distributed between 17.9 and 38, with a mean of 25.5 (Appendix Figure 3).
Table 1: Descriptive Statistic
|
Variable |
Mean |
Median |
SD |
Min |
Max |
|
Age (years) |
70.49 |
73.00 |
9.04 |
44.00 |
84.00 |
|
Height (cm) |
173.20 |
174.00 |
7.57 |
160.00 |
186.00 |
|
Weight (kg) |
79.86 |
83.00 |
15.05 |
46.00 |
115.00 |
|
BMI |
25.52 |
27.50 |
4.22 |
17.90 |
38.00 |
Figure 2: Distribution of height
Appendix Figure 1: Distribution of age
Appendix Figure 2: Distribution of weight
Appendix Figure 3: Distribution of BMI (body mass index)
To assess the reliability of the ECG method, the distance between the inserted CVC tips (Figure 1) was measured using the picture archiving and communication system (PACS). Across 51 patients, the mean difference between the two central lines inserted was 0.31 cm (Table 2). The median was 0.00 cm, indicating that more than half of the sample exhibited no difference in positioning. Collectively, the differences ranged from 0.00 cm to 1.00 cm.
Table 2: Overall difference between CVC tips. (Wilcoxon test with P-value < 0.001)
|
Variable |
Mean |
Median |
SD |
Min |
Max |
|
Difference (cm) |
0.31 |
0.00 |
0.39 |
0.00 |
1.00 |
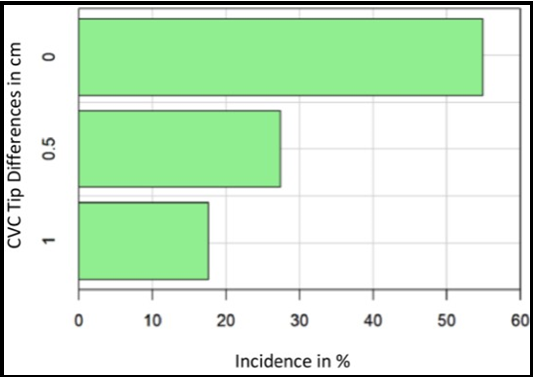
Percentage incidences of the CVC tip differences in the three groups (0.0 cm, 0.5 cm, and 1.0 cm) are illustrated in the bar chart (Figure 3) and show lower incidences of more significant differences.
The incidences of the various access site combinations and the corresponding differences in CVC tip placement are shown in Table 3. The incidences of access site combinations are remarkably heterogeneous, ranging from one (e.g., RIJV + RIJV, RSV + LIJV) to 10 (e.g., RIJV + LSV). The median and mean distances between the CVC tip positions show that the groups differ concerning CVC placement consistency. Groups with more considerable mean distances were LSV + RIJV (mean 1.0 cm), RIJV + LIJV (mean 0.67 cm), RSV + LIJV (mean 0.5 cm), and RIJV + LSV (mean 0.45 cm). However, no CVC tip difference (mean 0.00 cm) was observed in groups representing 16 % of the sample.
In all groups other than RIJV + LSV, the p-value was > 0.05, indicating that the CVC tip positions did not differ significantly.
Only the RIJV + LSV group showed p < 0.05. Here, the distance between the CVC tips was statistically significant, although the space was only 0.45 cm, which is clinically negligible. In the 16 exclusively right-sided combinations (31 %), no or almost no difference in CVC tip position was detected (mean 0.1 cm). In the 10 exclusively left-sided CVC combinations (20 %), the difference between CVC tips was minor (mean 0.25 cm). In contrast, the differences in the 25 crosswise venous access site combinations (49 %) were slightly more prominent (mean 0.46 cm) but still modest enough to imply no clinical consequence or disadvantage.
Figure 3: Incidence in % of CVC tip differences 0.00, 0.5 and 1.0 cm
CVC = central venous catheter
Table 3: Incidence of Combinations of various Access Sites and their corresponding Differences in CVC tips
|
Access Combinations |
n |
Mean |
Median |
SD |
P-value |
|
LIJV + LSV |
5 |
0.40 |
0.00 |
0.55 |
0.346 |
|
LSV + LSV |
5 |
0.10 |
0.00 |
0.22 |
1.000 |
|
LSV + RIJV |
3 |
1.00 |
1.00 |
0.00 |
0.149 |
|
RIJV + LIJV |
3 |
0.67 |
0.50 |
0.29 |
0.174 |
|
RIJV + LSV |
10 |
0.45 |
0.50 |
0.37 |
0.018 |
|
RIJV + RIJV |
1 |
0.00 |
0.00 |
- |
- |
|
RIJV + RSV |
8 |
0.19 |
0.00 |
0.26 |
0.149 |
|
RSV + LIJV |
1 |
0.50 |
0.50 |
- |
- |
|
RSV + LSV |
8 |
0.25 |
0.00 |
0.38 |
0.174 |
|
RSV + RIJV |
3 |
0.00 |
0.00 |
0.00 |
1.000 |
|
RSV + RSV |
4 |
0.00 |
0.00 |
0.00 |
1.000 |
CVC = central venous catheter
RIJV = right internal jugular vein
LIJV = left internal jugular vein
RSV = right subclavian vein
LSV = left subclavian vein
Discussion
The main finding of this study underlines the reliability and precision of the ECG method for placing CVC tips at P-max, where P-max illustrates the RA–SVC junction. In this case, 45/51 (88 %) ends of double central lines inserted differed only up to 0.5 cm each. The maximum difference between two lines placed at P-max was 1.00 cm in three patients (6 %), deemed clinically insignificant. Accordingly, the method should categorically be considered reliable. The literature was reviewed extensively for similar research, and no other study was found comparing tip positions of two central lines, i.e., chest X-ray, transthoracic/transoesophageal echocardiography, and the ECG method.
Why is the CVC tip position so important?
The quest for the ideal CVC tip position is still ongoing and involves balancing risk prevention on the one hand while ensuring the proper functioning of the catheter on the other. [16] The most worrisome risk is that of pericardial tamponade. [5,16] A common false assumption is that pericardial tamponade can only occur when the tip of a CVC enters the heart. [17] As we have learned from anatomical and computed tomography studies, the pericardial sac exhibits a high degree of anatomical variation. [18-21] In the typical clinical setting, we are confronted with an individual patient whose exact pericardial sac dimensions are unknown. Thus, recommendations to place the CVC tip outside the pericardial hazard zone lull us into a false sense of security. [10,16]
Moreover, CVCs placed outside the hazard zone and inserted too shallowly lead to other problems, such as intimal damage, thrombosis, tip migration, catheter malfunction, or vessel perforation. [4,6,8,10,16]
For the proper functioning of a CVC, flow through each lumina must not be impeded, and the running direction of the catheter must be parallel to the long axis of the SVC. [3,10,13,14,16] From a clinical point of view, the lower SVC near the RA–SVC junction may be the optimal position of the catheter tip for monitoring and administering vasoactive drugs during perioperative periods for the application of blood purification techniques. [4,10,12,16] This position seems to prevent malfunction, migration, and thrombosis. Moreover, if the catheter tip is positioned in the lower SVC near the RA–SVC junction, the catheter tip should float freely inside the vessel lumen without abutment and the devastating complication of cardiac tamponade may be avoided – even though the catheter tip is positioned below the level of pericardial reflection on the SVC and even in the RA. [4,10,16]
Good clinical practice demands verification of the catheter tip. Several methods can be considered. Techniques used during the catheter's placement are helpful for successful positioning and time- saving. Transoesophageal echocardiography (TOE) merits particular mention because it allows the position of the catheter tip to be judged about the RA–SVC transition. [10,12,15] However, this substantial technical effort is justified in only a few situations. Another technique is placement under real-time radiographic imaging; this approach is used only for permanent access procedures. [9,10] The easiest of the real-time techniques is demonstrably ECG guidance while advancing the catheter: when P-max is detected, the CVC tip is at the RA–SVC transition. [10,12,13,22] While this holds for patients in sinus rhythm, it may be difficult in patients with pacemakers or those in atrial fibrillation. [10,23]
The other verification methods – transthoracic echocardiography with its known limitations and bedside chest X-ray – are post-hoc techniques, the results of which are generally available only after finishing the placement procedure. [24,25] One of the tasks of postprocedural chest X-rays is to assess mechanical complications and malpositions; [9,25] another is to verify the course of catheters and their tips when other methods are unavailable or have failed. [9,25]
Simple calculation formulas do not have a place in the category verification technique. They provide us only with a long guess ahead of catheter placement. The relevance to the daily practice of formulas involving body height for predicting CVC insertion depth remains to be determined.
In a TOE-controlled study, a formula could only predict CVC position with sufficient precision. [26] Using the formula-based approach in individual patients thus entails accepting a considerable risk of catheter malpositioning. [26] Accordingly, this practice should be abandoned for safety reasons. [26]
Taking the importance of CVC tip position into account, the ECG method, as well as this study which intended to prove the reliability of the technique, was revealed in a special light. This is because the ECG method combines two features. First, the method enables the desired target position of a CVC tip at the RA–SVC transition. Second, detecting P-max offers reliability to the same site, the CVC tip, as demonstrated by this study.
Limitations
The time frame over which patients received their catheters ranged from both lines being inserted simultaneously to a lag of five days. Most catheters were placed two to three days apart. The variation in the time frame may have influenced the results. ECG guidance was realized via the Seldinger wire only. The "column of saline" method was not applied. Sticking to the guidewire technique may also be a factor for differences in positioning. Further, a chest X-ray is a momentary test offering only a snapshot of CVC tip positions at a specific time. However, migration of CVCs is possible at any time, e.g., due to respiratory movements or with the active or passive change in patient positioning. Central lines and their ECG-guided advancement to the P-max position were inserted with the patient lying supine. This position cannot be guaranteed for all bedside CXRs. Therefore, neither catheter migration nor parallax effects can be excluded. [15] However, these issues were deemed negligible because both central lines and their vertical tip difference were assessed using the same chest X-ray.
The ECG method has shown its functionality, especially in patients with sinus rhythm. However, this method works even in a substantial proportion of patients without sinus rhythm, although large-scale studies to support this finding are unfortunately unavailable. [23] Only patients in sinus rhythm participated in this study. Accordingly, our results are restricted to patients in sinus rhythm. In addition, catheters from left-sided access sites could not always be advanced to P-max because the maximum insertion length was only 21 cm. This and that measurements were performed using the Seldinger wire may explain the central issue of CVC tip differences, especially in the group exhibiting a difference of more than 0.50 cm. Each access group's wide variability and low numbers substantially restrict statistical analysis.
Conclusion
The ECG method of placing the CVC tip at P-max is a stable and reliable bedside method for positioning CVC tips and already works during the access procedure. Although demonstrated with only a few patients, the results for double CVCs are impressive from a clinical point of view and support the hypothesis. However, the results of this research demand confirmation in further studies
Support was provided solely from departmental sources.
Conflicts of Interest: none.
References
- McGee DC, Gould MK (2003) Preventing complications of central venous catheterization. N Engl J Med. 348(12): 1123–33.
- Schmidt GA, Blaivas M, Conrad SA, Corradi F, Koenig S, et al. (2019) Ultrasound-guided vascular access in critical illness. Intensive Care Med. 45(4): 434–446.
- Fletcher SJ, Bodenham AR (2000) Safe placement of central venous catheters: where should the tip of the catheter lie? Br J Anaesth. 85(2): 188–91.
- Vesely TM (2003) Central venous catheter tip position: a continuing controversy. J Vasc Interv Radiol. 14(5): 527–34.
- Booth SA, Norton B, Mulvey DA (2001) Central venous catheterization and fatal cardiac tamponade. Br J Anaesth. 87(2): 298–302.
- Cadman A, Lawrance JA, Fitzsimmons L, Spencer-Shaw A, Swindell R (2004) To clot or not to clot? That is the question in central venous catheters. Clin Radiol. 59(4): 349–55.
- Ballard DH, Samra NS, Gifford KM, Roller R, Wolfe BM, et al. (2016) Distance of the central venous catheter tip from the right atrium is positively correlated with central venous thrombosis. Emerg Radiol. 23(3): 269–73.
- Petersen J, Delaney JH, Brakstad MT, Rowbotham RK, Bagley CM (1999) Silicone venous access devices positioned with their tips high in the superior vena cava are more likely to malfunction. Am J Surg. 178: 38–41.
- Gibson F, Bodenham A (2013) Misplaced central venous catheters: applied anatomy and practical management. Br J Anaesth. 110(3): 333–46.
- Ahn JH, Kim IS, Yang JH, Lee IG, Seo DH, et al. (2017) Transoesophageal echocardiographic evaluation of central venous catheter positioning using Peres’ formula or a radiological landmark-based approach: a prospective randomized single- centre study. Br J Anaesth. 118(2): 215–222.
- Robinson JF, Robinson WA, Cohn A, Garg K, Armstrong JD (1995) Perforation of the great vessels during central venous line placement. Arch Intern Med. 155(11): 1225–8.
- Chu KS, Hsu JH, Wang SS, Tang CS, Cheng KI, et al. (2004) Accurate central venous port-A catheter placement: intravenous electrocardiography and surface landmark techniques compared by using transesophageal echocardiography. Anesth Analg. 98(4): 910–914.
- Schummer W, Schummer C, Schelenz C, Schmidt P, Fröber R, et al. (2005) Modified ECG-guidance for optimal central venous catheter tip positioning. A transesophageal echocardiography controlled study. Anaesthesist. 54(10): 983–90.
- Kremser J, Kleemann F, Reinhart K, Schummer W (2011) Optimized method for correct left-sided central venous catheter placement under electrocardiographic guidance. Br J Anaesth. 107(4): 567–72.
- Wirsing M, Schummer C, Neumann R, Steenbeck J, Schmidt P, et al. (2008) Is traditional reading of the bedside chest radiograph appropriate to detect intraatrial central venous catheter position? Chest. 134(3): 527–533.
- Tempe DK, Hasija S (2017) Quest to determine the ideal position of the central venous catheter tip. Br J Anaesth. 118(2): 148–150.
- Food-and-Drug-Administration (1989) Precautions necessary with central venous catheters. FDA Task Force. FDA Drug Bulletin: 15–16.
- Kwon TD, Kim KH, Ryu HG, Jung CW, Goo JM, et al. (2005) Intra- and extra-pericardial lengths of the superior vena cava in vivo: implication for the positioning of central venous catheters. Anaesth Intensive Care. 33(3): 384–7.
- Caruso LJ, Gravenstein N, Layon AJ, Peters K, Gabrielli A (2002) A better landmark for positioning a central venous catheter. J Clin Monit Comput. 17(6): 331–4.
- Bayer O, Schummer C, Richter K, Fröber R, Schummer W (2006) Implication of the anatomy of the pericardial reflection on positioning central venous catheters. J Cardiothorac Vasc Anesth. 20(6): 777–80.
- Dulce M, Steffen IG, Preuss A, Renz DM, Hamm B, et al. (2014) Topographic analysis and evaluation of anatomical landmarks for placement of central venous catheters based on conventional chest X-ray and computed tomography. Br J Anaesth. 112(2): 265–71.
- Ender J, Erdoes G, Krohmer E, Olthoff D, Mukherjee C (2009) Transesophageal echocardiography for verification of the position of the electrocardiographically-placed central venous catheter. J Cardiothorac Vasc Anesth. 23(4): 457–61.
- Steinhagen F, Kanthak M, Kukuk G, Bode C, Hoeft A, et al. (2018) Electrocardiography-controlled central venous catheter tip positioning in patients with atrial fibrillation. J Vasc Access. 19(6): 528–534.
- Vezzani A, Brusasco C, Palermo S, Launo C, Mergoni M, et al. (2010) Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 38(2): 533–8.
- Raptis DA, Neal K, Bhalla S (2020) Imaging Approach to Misplaced Central Venous Catheters. Radiol Clin North Am. 58(1): 105–117.
- Struck MF, Schmidt T, Winkler BE, Reinhart K, Schummer W (2015) Central venous catheters and insertion depths: are formulas still up to date? Intensive Care Med. 41(11): 2002–3.