Alkistis Adramerina MD PhD*, Anna Taparkou BS Msc, Pavlos Siolos MD, Sofia Karagiannidou MD Msc, Marina Economou MD PhD
1st Pediatric Department, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
*Corresponding Author: Alkistis Adramerina MD PhD, 1st Pediatric Department, School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Abstract
Neonatal immune thrombocytopenia may occur secondary to any maternal autoimmune disease. We report on a case of severe thrombocytopenia presenting in a neonate born to a mother with a history of aplastic anemia. The cytopenia was unresponsive to multiple courses of high dose intravenous immunoglobulin, finally demonstrating spontaneous – even though delayed – recovery without in between bleeding complications.
Keywords: neonatal thrombocytopenia; immune thrombocytopenia; aplastic anemia
1. Introduction
Thrombocytopenia is the most common cytopenia during the neonatal period, presenting in 1-5% of newborns at birth. Platelet count below 50 x 109/L is considered as severe thrombocytopenia and concerns 0.1-0.5% of all neonatal cases [1]. The causes of thrombocytopenia vary and are related either to pediatric pathological conditions or to disorders at the level of the fetal-placenta-maternal interface [2,3].
Theoretically, neonatal immune thrombocytopenia may occur in any maternal autoimmune disease. The intrauterine onset indicates maternal antibodies targeting both maternal and fetal platelets, while onset within the first 72hours of life results from transplacental passage of maternal antibodies [2,3]. In the case of maternal immune thrombocytopenia, a low platelet count may be found in up to 10% of neonates. Most reports concern neonatal cases with mild to moderate thrombocytopenia, usually characterized by an uncomplicated course [1]. Severe thrombocytopenia leading to major bleeding, such as intracranial haemorrhage, is rare - estimated at less than 1% of cases [4].
Aplastic anemia is a disorder of the hematopoietic stem cell that results in bone marrow failure. The precise pathogenic mechanism, though unclear, is likely to be immune-mediated, with the implication of activated cytotoxic T cells resulting in cytokine overproduction and apoptosis induction [5,6]. Pregnancy in women with aplastic anemia is rare and associated with serious maternal and fetal risks [6].
We herein report on a case of persistent severe thrombocytopenia presenting in a neonate born to a mother with a history of aplastic anemia.
2. Case Description
A male neonate, 16 days of age, was admitted to our pediatric hematology department due to persistent thrombocytopenia presenting at birth. The baby was the first born of a mother with a history of aplastic anemia. He was delivered preterm (33+4 weeks) due to an abnormal fetal nonstress test, but it was appropriate for gestational age and presented with no phenotypic abnormalities.
With regards to the mother’s medical history, she was born to a mother with systematic lupus erythematosus (SLE) and presented with thrombocytopenia during the first days of her life. Reportedly, her thrombocytopenia was successfully treated with immunoglobulin. According to the scarce available data, at the age of 2.5 years, she was diagnosed with non-severe aplastic anemia with prominent thrombocytopenia. Bone marrow biopsy at diagnosis showed hypocellular bone marrow without morphological evidence of leukemia or myelodysplasia. Multiple therapies, including immunoglobulin, corticosteroids, and cyclosporin, were administered before she was referred to a specialized pediatric hematology department abroad for further treatment.
Normalization of leucocyte count and hemoglobin levels at the age of 17 was reported with maintenance of an almost normal platelet count (~ 100 x 109/L) ever since. No underlying autoimmune disease was established by her treating physicians during the years that followed.
As for her baby boy, he was hospitalized in a neonatal unit for the first two weeks of his life due to prematurity and severe thrombocytopenia (30-50 x 109/L); however, with no reported bleeds or other complications. The little patient was hospitalized till full enteral feeding was achieved, showing no other complication of prematurity or need for interventions.
Following referral to our department, initial work up showed a white blood cell count of 16800/mL, a hemoglobin level of 11g/dl and a platelet count of 47 x 109/L. No signs of hemorrhage were evident in the otherwise healthy, breast fed baby. Direct antiglobulin test was negative and abdomen ultrasound was normal. A TORCH infection was excluded, as well as neonatal alloimmune thrombocytopenia with Human-Platelet-Antigen-1 (HPA-1) genotyping in mother and child. Anti-platelet antibody presence using flow cytometry was positive, revealing IgG and IgM antibodies in both mother and child. The rest immunological work up, including antinuclear antibodies (ANA), anti-DNA antibodies, immunoglobulins (Ig) A, G, M, as well as C3 and C4 revealed no pathological findings.
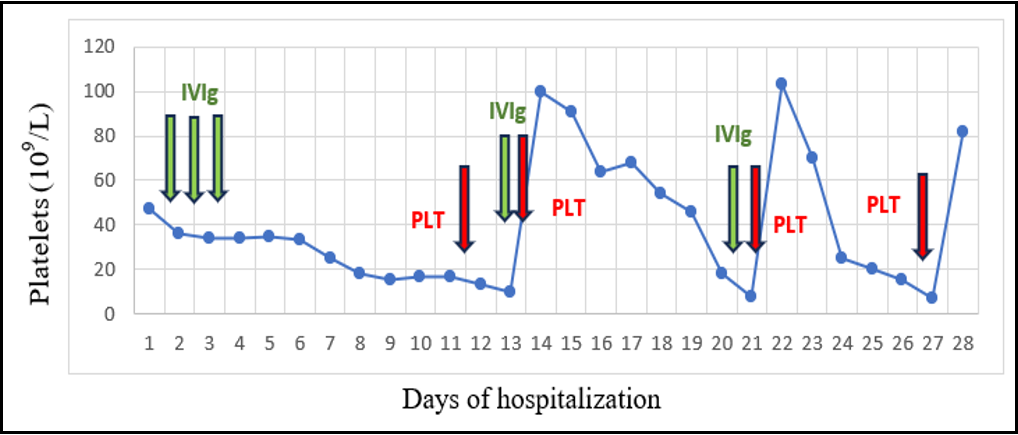
After referral, the patient’s platelet count gradually decreased to a level of below 20 x 109/L. Courses of intravenous immunoglobulin were repeatedly administered without response. Besides occasional petechiae, the baby also presented with an incident of bloody stools, for which he received a platelet transfusion. (Table 1)
Table 1: Platelet count distribution during patient’s hospitalization.
Due to his good general condition and the absence of other bleeding episodes, the baby’s discharge was decided at the age of 45 days, with a recommendation for close follow up. During the weeks that followed, he required platelet transfusions every 1-2 weeks in order to maintain a relatively safe platelet count of approximately 20-30 x 109/L. The anti-platelet antibody presence continued at a similar level in both mother and child.
At the age of 3 months the baby’s platelet count stabilized and then slowly begun to rise, until becoming normal after approximately 2 months. In the meantime, the IgG anti-platelet antibodies became non-detectable, while the IgM antibodies remained positive. At that point the family opted for discontinuation of follow up.
The patient showed up, though, 2 years later presenting normal blood count as well as absence of anti-platelets antibodies. His mother maintained a platelet count around 100 x 109/L without anti-platelets antibodies anymore.
3. Discussion
Neonatal thrombocytopenia is a common finding, especially in critically ill full- or preterm neonates, having a broad range of possible etiologies [2]. Fortunately, most cases of neonatal thrombocytopenia are mild or moderate and resolve without intervention, thrombocytopenia often being an incidental finding on laboratory tests being performed for other indications [1]. The present case report concerns a preterm neonate diagnosed with thrombocytopenia during his hospitalization in a neonatal unit and, even though the cytopenia was not associated with a clinically significant bleeding tendency, it persisted long after the first few weeks of life.
Thrombocytopenia at birth secondary to maternal idiopathic thrombocytopenic purpura or systemic lupus erythematosus is well described [2,7]. Neonatal thrombocytopenia may occur not only due to maternal active immune disease, but also at states of remission [2]. Markers of maternal autoimmune disease, such as platelet count or antibody level, have failed to predict a newborn’s platelet count at birth, and the only major predictor is a prior history of severe neonatal thrombocytopenia in a sibling [2,4].
To our knowledge this is the first report of neonatal immune thrombocytopenia in the context of maternal aplastic anemia. The patient’s mother was a long-term survivor in complete remission since the 17th year of life. Although aplastic anemia was considered a fatal disease in the past, its outcome has improved since 1970, and even more in recent years, due to immunosuppressive treatment (IST) and hematopoietic cell transplantation. Recently, Dexler et al. reported an overall survival of patients with aplastic anemia of approximately 40% at 30 years, with the majority of long term survivors in complete remission, irrespective of initial treatment modality [8]. Furthermore, blood count normalization in IST treated patients may be delayed and long-term cyclosporine therapy required in a subgroup of patients, due to the autoimmune character of the disease [9]. Positive maternal anti-platelet antibodies were indicative of the underlying immune mechanism, even if the mother demonstrated adequate balance between platelet destruction and platelet production, maintaining a platelet count of >100 x109/L.
In cases of immune thrombocytopenia at birth, the lowest platelet count is known to occur in the first 24 hours up until the first 2 weeks of life. Even though the laboratory finding may persist for up to 4 months, the bleeding risk is considered minimal after the first 2 weeks of life [2]. In the case herein presented, the platelet count reached its lowest level later than anticipated. Similarly, platelet count recovery showed a delay - finally occurring without bleeding episodes of clinical significance in between.
In neonatal immune thrombocytopenia intravenous immunoglobulin is considered the treatment of choice, with success rates that reach 90%. Corticosteroid effectiveness as an alternative treatment has not been confirmed [2]. In the case described, administration of high dose IVIg was not sufficient to maintain an adequate platelet count. To that end, platelet transfusions were decided so as to enable discharge, even if slowing down course to recovery.
Of interest, IgG anti-platelet antibodies ceased to be detected in the infant during follow up and platelet count normalized, even though IgM anti-platelet antibodies persisted long after breast feeding discontinuation. Given the genetic predisposition that characterizes aplastic anemia, questions on the child’s final prognosis - and diagnosis - still remains.
Conflict of Interest: The authors declare no conflict of interest.
References
- Roberts I, Murray NA (2003) Neonatal thrombocytopenia: causes and management. Arch Dis Child Fetal Neonatal Ed. 88(5): F359- 64.
- Donato H (2021) Neonatal thrombocytopenia: A review. I. Definitions, differential diagnosis, causes, immune thrombocytopenia. Arch Argent Pediat. 119(3): e202-e214.
- Sillers L, Van Slambrouck C, Lapping-Carr G (2015) Neonatal Thrombocytopenia: Etiology and Diagnosis. Pediatr Ann. 44(7): e175-80.
- Koyama S, Tomimatsu T, Kanagawa T, Kumasawa K, Tsutsui T, et al. (2012) Reliable predictors of neonatal immune thrombocytopenia in pregnant women with idiopathic thrombocytopenic purpura. Am J Hematol. 87(1): 15-21.
- Young NS (2018) Aplastic Anemia. N Engl J Med. 379(17): 1643-1656.
- Shin JE, Lee Y, Kim SJ, Shin JC (2014) Association of Severe Thrombocytopenia and Poor Prognosis in Pregnancies with Aplastic Anemia. PLoS One. 9(7): e103066.
- Beryl S, Ross BJ, Tergestina M, Kumar M (2021) Severe neonatal autoimmune thrombocytopenia secondary to maternal Evans syndrome. BMJ Case Rep. 14(12): e245695.
- Drexler B, Zurbriggen F, Diesch T, Viollier R, Halter JP, et al. (2020) Very long-term follow-up of aplastic anemia treated with immunosuppressive therapy or allogeneic hematopoietic cell transplantation. Ann Hematol. 99(11): 2529-2538.
- Frickhofen N, Heimpel H, Kaltwasser JP, Schrezenmeier H (2003) Antithymocyte globulin with or without cyclosporin A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood. 101(4): 1236-42.