Radomir Damjanovic1*, Srdjan Ljubisavljevic1,2, Ana Prazic1, Lilika Zvezdanovic3
1Clinic for Neurology, University Clinical Center of Nis, Nis, Serbia
2Faculty of Medicine, University of Nis, Nis, Serbia
3Center for Biochemistry, University Clinical Center of Nis, Nis, Serbia
*Corresponding Author: Radomir Damjanovic, MD, Clinic for Neurology, University Clinical Center of Nis, Nis, Serbia
Abstract
This is a case of Paget's disease of bone (PDB) with a quick, progressive clinical course, initially presented with neurological symptoms, which is rare in previously shown literature. Also, it occurred in a region unusual for PDB. This diagnosis should be considered in the clinical states of an impressive neurological presentation like the one.
Keywords: Paget's disease of bone (PDB), neurological symptoms, bone turnover, alkaline phosphatase, bisphosphonates
Introduction
The second-most common metabolic disorder of the bone, after osteoporosis, is Paget's disease of bone (PDB), also known as osteitis deformans. An osteoclast defect causes more significant bone destruction, prompting osteoblastic new bone production. Because the natural balance between the two processes is impaired, this condition is characterized by an unorganized bone turnover [1]. As pagetic bone is more vascular, less compact, and enlarged, it is more prone to deformities and fractures. Bone or joint pain, fractures, bone abnormalities and enlargement, and even malignant transformation are common symptoms and manifestations [2]. Elevated serum alkaline phosphatase, distinctive radiographic features, and bone nuclear scintigraphy are used to diagnose it [1].
There are monostotic and polyostotic forms of PDB, with the former being the more prevalent [3]. Typically, PDB appears in people who are middle-aged or older. According to post-mortem and radiographic investigations, the prevalence of PDB is 3% to 3.7% worldwide [4,5,6]. Great Britain, Australia, New Zealand, North America, and Western Europe have the most excellent prevalence rates [7,8].
Case Report
A 67-year-old female was admitted to our Clinic due to double vision, bilateral deafness, and progressive skull deformation. She stated that a double vision had been present for one month. In that period, her hearing became progressively worse. From family medical history, we found that her father had Parkinson's disease, and her brother has bilateral deafness. Her mother had polydactyly.
Physical examination showed craniomegaly with diffuse alopecia (Figure. 1a and 1b). The neurological study showed sixth cranial nerve palsy on the right, hypotension on both sides and a waddling manner of walking.
Figure 1a and 1b: Craniomegaly with diffuse alopecia
Laboratory reports revealed a progressive increase in the serum alkaline phosphatase (1120 U/L, Normal range 30-120 U/L) lactate dehydrogenase (455 U/L, Normal range 100-190 U/L), while serum calcium and phosphorus were normal. Urine calcium concentration in a 24-hour sample was decreased, while the concentration of urine phosphorus was average.
Since Multiple myeloma and PDB show similarities such as increased osteoclastic activity and bone resorption, serum protein electrophoresis was performed, which showed moderate elevation of alpha 2 fraction (13.4%, Normal range 7-11%). Magnetic resonance (MR) of the endocranium showed diffuse thickening of calvarial bones and skull base, with pons compression by thickened clivus and suspected compression of the sixth cranial nerve (Figure 2a, b, and c). Direct pelvis X-ray showed osteosclerosis of pelvic bones and proximal halves of both femurs. Skull deformities, deafness, radiology findings, and increased serum alkaline phosphatase were sufficient for diagnosing PDB. Serum parathyroid hormone level was more than twice as high as the upper limit of the reference range (132.1 pg/mL, normal range 10-65 pg/mL), while Vitamin D was lower (3.4, normal range >30 ng/mL). Specific marker of bone resorption, serum beta – Cross Laps was 2.3 pg/mL (normal range 50- 450 pg/mL). Treatment was commenced with a single intravenous infusion of 5 mg zoledronic acid associated with oral calcium intake.
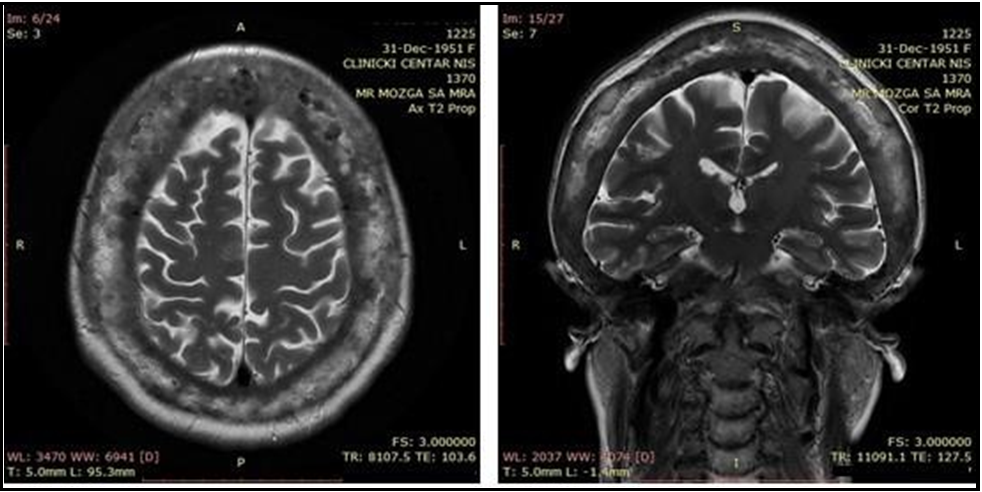
Figure 2a and 2b: MR of endocranium - axial section (a), and coronal section (b)
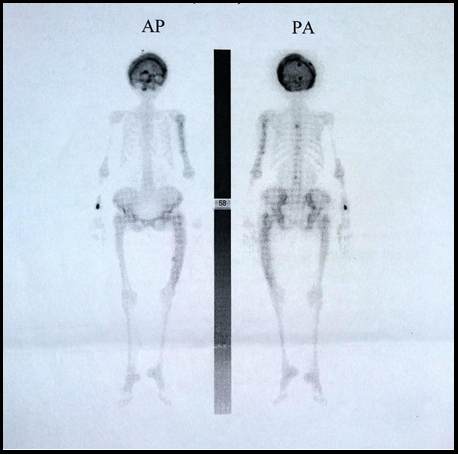
After being discharged from the hospital treatment, her symptoms worsened within a month. There was a ptosis of the right eyelid, paresthesia of the right half of the face, and mild to occasionally severe headache. The neurological examination also showed total ophthalmoplegia on the right side with mydriasis. She was admitted to our Clinic for the second time. Serum alkaline phosphatase was fifteen times higher than the upper limit value (1930 U/L), while serum phosphorus and calcium were average. Repeated MR imaging did not show a significant difference concerning the previous finding. Ill-defined lucent and sclerotic areas, as well as thickening of the calvarium, were presented on skull x-rays. Bone scintigraphy with 99mTc-DPD demonstrated increased diffuse uptake in the calvarial, parietal, and supraorbital parts of the frontal bone (Figure 3). The patient was treated with symptomatic therapy.
Figure 3: Bone scintigraphy with 99mTc-DPD
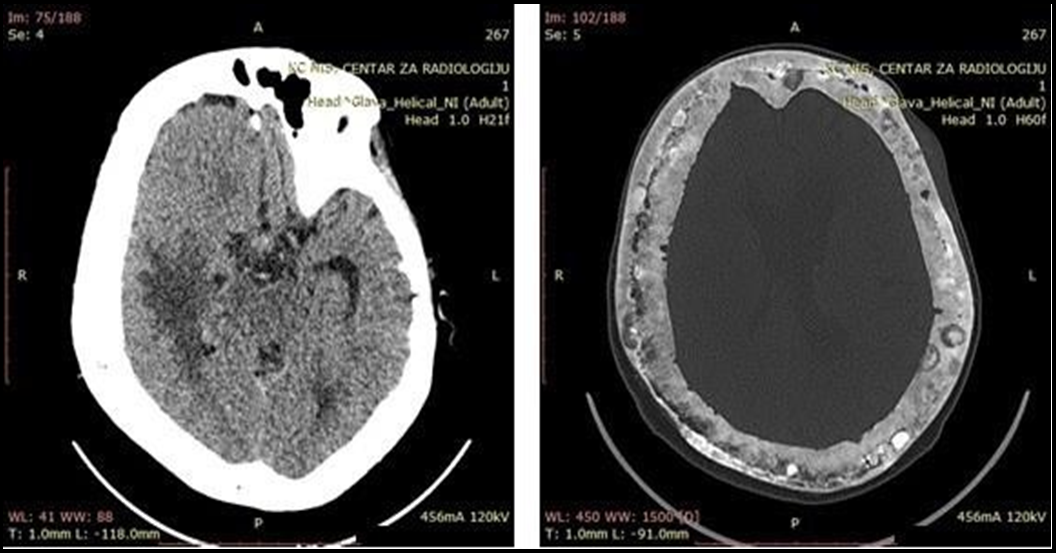
Further, in the next month, due to a neurologic worsening, the patient was admitted again to our Clinic. Neurological findings showed previous residual results with the appearance of a left-side pyramidal deficiency. The serum alkaline phosphatase was 2059 U/L. Computerized tomography (CT) of the endocranium showed diffuse lytic bone lesions and multiple neoplasm-like structures infiltrating brain parenchyma (Figure 4). The symptomatic therapy was applied.
Discussion
PDB is expected in the Caucasian Population [9]. Based on the available literature, PDB appears rare in the Balkans and Serbia. The usual course of PDB has three phases [10]. The first phase is osteolytic, the second is mixed osteoblastic/osteolytic, where the mineralization of new bone matrix is ineffective, and the third is sclerotic [10]. At the time of first admission, our patient was in the second phase with dominantly osteoblastic hyperplasia, according to the magnetic resonance, direct X-ray, and bone scintigraphy.
Common initial symptoms of the disease include bone pain, bone deformities, symptoms of fractures, decreased hearing, and headache [1]. Our patient was admitted to our Clinic because of undefined diplopia in the first line. Although neurologic symptoms are uncommon, their appearance is possible during the evolution (exacerbation) of the disease [11]. However, according to the literature, the condition has been rarely previously reported to manifest with neurological presentation and diplopia initially. Diagnosis is based on characteristic radiographic findings and by nuclear scintigraphy of the bone [1]. Magnetic resonance (MR) of the endocranium showed diffuse thickening of calvarial bones and skull base and compression of the sixth cranial nerve, which could be the reason for six nerve palsy. Bone scintigraphy demonstrated increased
diffuse uptake in the calvarial, parietal, and supraorbital parts of the frontal bone as a confirmation of the finding of the skull X-ray. Scintigraphy has a vital role in the diagnosis of PDB because of its high sensitivity for the detection of increased osteoblastic activity [13]. The biochemical markers of this disease are numerous, but one of the most commonly used is serum alkaline phosphatase, which reflects osteoblast activity [1]. Our patient showed high serum alkaline phosphatase levels at admission to our Clinic, while serum calcium and phosphorus were normal. The alkaline phosphatase can also be considered a sensitive marker for the therapeutic monitoring of PDB [9]. Urinary calcium is an indicator of bone resorption [9]. The concentration of calcium in a 24-hour urine sample was decreased, which also confirms the second stage of the disease.
Remedies of choice for the treatment of this disease are bisphosphonates, which reduce osteoclast-induced resorption and osteoclastic maturation [13]. Furthermore, bisphosphonates achieve a rapid reduction in bone turnover in PDB by inhibiting osteoclast resorption [14]. The treatment started with a single intravenous infusion of 5 mg zoledronic acid associated with oral calcium. Patients should be followed by measuring bone markers every three to six months depending on the activity of the pagetic lesions and the drug used [15]. However, in our patient, the applied therapy favors osteoblastic action, which can cause expansive changes of the calvarial bone that exert pressure on the brain tissue in this way, leading to neurological worsening (Figure 4).
This is a case of PDB with a quick, progressive clinical course, initially presented with neurological symptoms, which is rare in previously shown literature. Also, it occurred in a region unusual for PDB. This diagnosis should be considered in the clinical states of a unique neurological presentation such as the one presented here.
Figure 4a and 4b: Computerized tomography (CT) of endocranium
Conflict of interest: The authors declare no conflict of interest.
Acknowledgements: We want to thank the patient and her family for their consent for this publication.
References
- Kravets I (2018) Paget's Disease of Bone: Diagnosis and Treatment. The American Journal of Medicine. 131(11): 1298– 1303.
- Whyte M.P (2006) Paget’s Disease of Bone. NEJM. 355(6): 593- 600.
- Shankar YU, Misra SR, Vineet DA, Baskaran P (2013) Paget disease of bone: A classic case report. Contemp Clin Dent. 4(2): 227-230.
- Collins DH (1956) Paget’s disease of bone: incidence and subclinical forms. Lancet. 271(6933): 51-7.
- Schmorl G (1932) Uber osteitis deformans Paget. Virch Arch Anat Physiol. 183: 694-701.
- Pygott F (1957) Paget’s disease of bone: the radiological incidence. Lancet. 272(6980): 1170-1.
- Doyle T, Gunn J, Anderson G, Gill M, Cundy T (2002) Paget’s disease in New Zealand: evidence for declining prevalence. Bone. 31(5): 616-619.
- van Staa TP, Selby P, Leufkens HG, Lyles K, Sprafka JM, et al. (2002) Incidence and natural history of Paget’s disease of bone in England and Wales. J Bone Miner Res. 17(3): 465-471.
- Hadjipavlou AG, Gaitanis IN, Kontakis GM (2002) Paget's disease of the bone and its management. J bone Joint Surg (Br). 84(2): 160-169.
- Schneider D, Hofmann MT, Peterson JA (2002) Diagnosis and treatment of Paget’s disease of bone. Am Fam Physician. 65(10): 2069-2073.
- Feldman RG, Culebras A, Schmidek HH (1979) Paget’s disease and the nervous system. J Am Geriatr Soc. 27(1): 1-8.
- Kumar AA, Kumar P, Prakash M, Tewari V, Sahni H, et al. (2013) Paget's disease diagnosed on bone scintigraphy: Case report and literature review. Indian J Nucl Med. 28(2): 121-123.
- Papapoulos SE (1997) Paget's disease of bone: clinical, pathogenetic and therapeutic aspects. Bailliere’s Clinical Endocrinology and Metabolism. 11(1): 117-143.
- Siris ES, Lyles KW, Singer FR, Meunier PJ (2006) Medical management of Paget’s disease of bone: indications for treatment and review of current therapies. J Bone Miner Res. 21(2): 94-98.
- Altman RD (1987) Musculoskeletal manifestations of Paget’s disease of the bone. Arthritis Rheum. 23(10): 1121-7.