Sandra Belalcázar-Rey1*, Maria Elisa Mejía2, Jorge Escobar-DiazGranados3, Ángela María Ruiz-Sternberg4.
1Escuela de Medicina y Ciencias de la Salud, Universidad del Rosario, Carrera, 24 No 63C - 69 Barrio Siete de Agosto, Bogotá, DC, Colombia.
Fundación Oftalmológica Nacional, Calle 50 # 13-50, Bogotá, DC, Colombia. ORCID: 0000-0002-8645-8885
2Escuela de Medicina y Ciencias de la Salud, Universidad del Rosario. Fundación Oftalmológica Nacional, Calle 50 # 13-50, Bogotá, DC, Colombia. ORCID: 0000-0003-2483-2234
3Escuela de Medicina y Ciencias de la Salud, Universidad del Rosario. ORCID: 0000-0003-0259-276X
4.Escuela de Medicina y Ciencias de la Salud, Universidad del Rosario. ORCID: 0000-0002-4651-4635
*Corresponding Author: Sandra Belalcázar-Rey, Escuela de Medicina y Ciencias de la Salud, Universidad del Rosario, Carrera, 24 No 63C - 69 Barrio Siete de Agosto, Bogotá, DC, Colombia. Fundación Oftalmológica Nacional, Calle 50 # 13-50, Bogotá, DC, Colombia. ORCID: 0000-0002-8645-8885
Abstract
Background:
Intracranial compressive lesions masquerading as glaucomatous optic neuropathy have been previously described in the literature but remain a diagnosis challenge for trained physicians. We present a case series of patients referred to our center as glaucoma suspects or diagnosed with glaucoma, looking for a second opinion. We aim to highlight how clinical clues and Optical Coherence Tomography (OCT) findings helped identify a condition different from glaucoma.
Case presentation:
A 55-year-old male presented a painless decrease in his visual acuity and elevated intraocular pressure of both eyes. His OCT revealed Ganglion Cell Layer thinning, and Humphrey's visual field showed temporal hemianopia of the left eye. The second case shows a 38-year-old male referred for glaucoma evaluation due to cupping of the optic nerves. His OCT evidenced thinning of the optic nerves and macula. Humphrey's visual field revealed defects on both eyes that respected the vertical meridian. Both cases required neuroimaging to identify a neurological disease involving the visual pathway. The third case shows a 55-year-old male referred to as a glaucoma suspect due to thinning of the Retinal Nerve Fiber Layer and Ganglion Cell Layer. After correlating his clinical examination with the results of neuroimages, OCT, and visual fields, a trans neuronal retrograde degeneration was suggested due to his previous neurological history. The fourth case shows a 44-year-old male diagnosed with glaucoma looking for a second opinion after a surgical procedure was suggested due to cupping of his optic nerves. The changes in his optic nerves, OCT, and visual fields were related to a Pituitary Adenoma previously resected.
Conclusion:
A thorough clinical examination with an adequate interpretation of diagnostic tools may help differentiate neurological lesions compromising the visual pathway from glaucoma. Neuroophthalmological diseases must be carefully considered in the workup of patients with a glaucomatous appearance of the optic nerve, without a typical correlation of structure-function tests such as OCT and visual fields.
Keywords: Neuroophthalmological diseases, Intracranial compressive lesions, Glaucomatous optic neuropathy, Optical Coherence Tomography, Clinical examination.
Introduction
Glaucomatous optic neuropathy is a multifactorial neurodegenerative disease characterized by a loss of retinal ganglion cells and their axons. This results in the alteration of the optic nerve head topography, retinal nerve fiber layer loss, and deterioration of the visual function [1-3]. Different vascular, compressive, or hereditary neuroophthalmological diseases may resemble glaucomatous presentation in general practice, which can be misdiagnosed as normal-tension glaucoma [4]. Differentiation between glaucomatous and non-glaucomatous disc cupping is often challenging for trained physicians, especially when the intraocular pressure (IOP) is within the normal range [3,4]. New technologies such as Spectral-Domain Optical Coherence Tomography (SD-OCT) have enabled the measurement of the circumpapillary Retinal Nerve Fiber Layer (RNFL) and macular Retinal Ganglion Cell (RGC) thicknesses, which complements the clinical examination of the optic disc by detecting retinal abnormalities resulting from axonal loss [1-3]. Even though OCT measured patterns of RNFL thinning in compressive optic neuropathies may mimic those in glaucoma, a complete clinical history, examination, and presentation of visual field defects could help differentiate between diseases [3].
The incorrect assessment of neuroophthalmological diseases may lead to scenarios of late diagnosis of treatable intracranial tumors or unnecessary treatment [2-4]. This means that differentiation between glaucomatous and non-glaucomatous optic neuropathies is both clinically and economically relevant [2-4]. Although neuroophthalmological diseases mimicking glaucoma have been previously reported, masqueraders of glaucomatous optic neuropathy still provide diagnostic challenges for clinicians [3,4]. Keeping this in mind, we aim to highlight the value of clinical examination and adequate interpretation of diagnostic tools, which helped differentiate a neurological pathology from glaucoma. We present a four-case series referred to our center as glaucoma suspects or diagnosed with glaucoma, looking for a second opinion.
Patients Information:
Case 1:
A 55-year-old Hispanic male was referred to our center as a glaucoma suspect due to a painless decrease in his visual acuity and elevated IOP in both eyes in November 2015. His family, medical and ocular history was unremarkable. His best-corrected visual acuity was 20/20 in his right eye (OD) and 20/100 in his left eye (OS). IOP ranged between 19-24 mmHg in both eyes, on no medications; Corneal pachymetry fell within the normal range for both eyes. An afferent pupillary defect in the left eye was identified and an Amsler test evidenced blurred vision at the central fixation point in the left eye. Gonioscopy showed open angles in both eyes. Slit-lamp examination was unremarkable, and optic nerve evaluation showed cup-to-disc ratios of 0.4 and 0.6 OD and OS, respectively (Figures 1A and 1B).
Two months later, the patient was re-evaluated with results of SD- OCT imaging that revealed normal RNLF thickness in both eyes and macular GCL thinning in the inferior retina of the right eye, and GLC thinning in all sectors of the left eye (Figure 1C and 1D). Humphrey's visual field testing showed no significant defects in the right eye and temporal hemianopia of the left eye (Figure 1E and 1F). An MRI was performed and evidenced a Tuberculum Sellae Meningioma compromising the visual pathway (Figure 2). In April 2016, the resection of the tumor was performed using a transcranial approach with no complications. In January 2017, the patient's best-corrected visual acuity returned to 20/20. Humphreys’ visual field test in 2017 and 2018 evidenced progressive recovery of the visual fields (Figure 3).
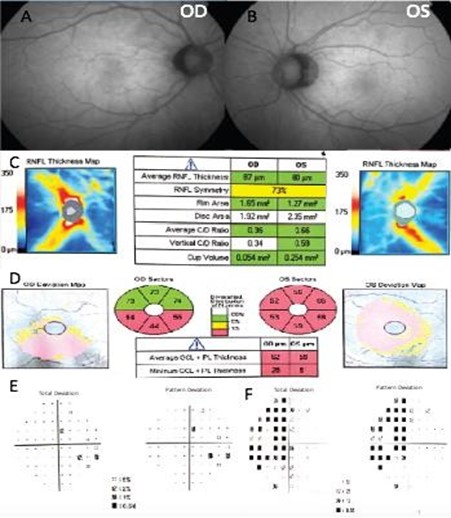
Figure 1. Fundus photography of the right eye (A) and left eye (B). The spectral-domain optical coherence tomography (SD-OCT), peripapillary retinal nerve fiber layer thickness analysis (C), and ganglion cell macular thickness analysis (D). Humphrey visual field of the right eye (E) and left eye (F).
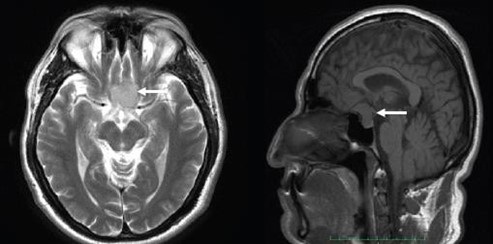
Figure 2. Axial and sagittal cuts of an MRI evidencing Tuberculum Sellae Meningioma (white arrows).
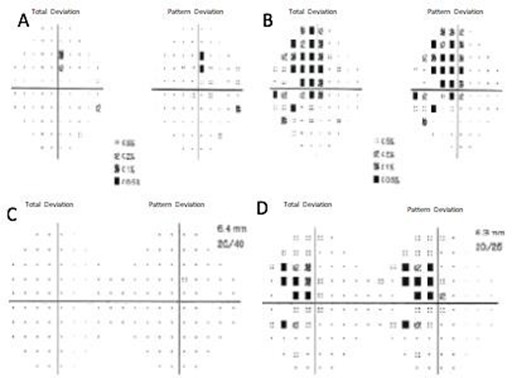
Figure 3. 2017 Humphrey visual field of the right eye (A) and the left eye (B). 2018 Humphrey visual field of the right eye (C) and the left eye (D).
Case 2:
A 38-year-old Hispanic male was referred for glaucoma evaluation due to cupping of the optic nerves in August 2016. His medical history showed that he suffers from Gilbert’s syndrome. The patient also referred first-degree family members with age-related macular degeneration, nyctalopia, and pigmentary retinosis. His visual acuity measured 20/20 in each eye, and color vision was normal. His intraocular pressures were in the low teens (13-14 mmHg) in both eyes, on no medications; central corneal thickness was 528 microns OD and 525 microns OS. Gonioscopy showed open angles in both eyes. Slit-lamp examination was unremarkable, and optic nerve evaluation showed cup-to-disc ratios of 0.5 and 0.6 OD and OS, respectively (Figure 4A and 4B).
He brought an SD-OCT that revealed inferior, superior, and temporal thinning of the RNFL of the right eye, and macular thickness was reduced in all sectors. In the left eye, superior thinning of the RNFL was evidenced, and macular thickness was severely reduced in the Hemi nasal retina (Figure 4C and 4D). Humphrey's visual field testing showed an inferior nasal cuadrantanopsy of the right eye, and a temporal defect that respects the vertical line in the left eye (Figure 4E and 4F). We ordered an MRI and evaluation with a neurosurgeon. Four months later, he was evaluated with the MRI results that evidenced an aneurysmal formation of the right supraclinoid carotid siphon with a medial posteroinferior orientation of cavernous angioma (Figure 5). The neurosurgeon considered that the lesion had remained stable over time and did not require surgical management.
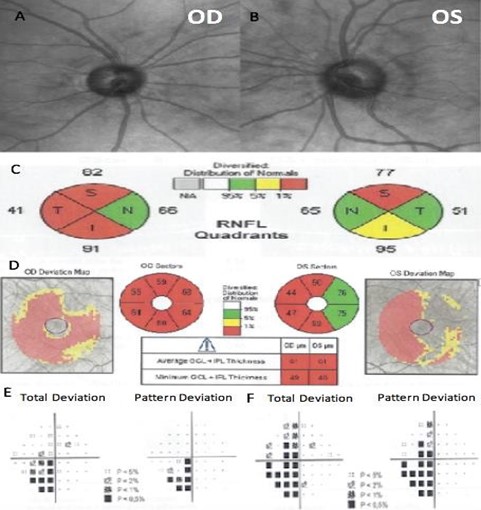
Figure 4. Fundus photography of the right eye (A) and left eye (B). The spectral-domain optical coherence tomography (SD-OCT) peripapillary retinal nerve fiber layer thickness analysis by quadrants (C), and ganglion cell macular thickness analysis (D). Humphrey visual field of the right eye (E) and left eye (F).
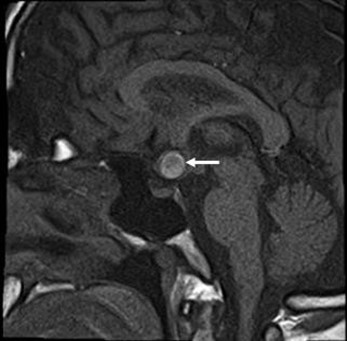
Figure 5. Cavernous Hemangioma with a sellar extension (white arrow).
Case 3:
In February 2018, a 55-year-old Hispanic male was referred to our clinic as a glaucoma suspect due to thinning of the RNFL and GCL in the Optical Coherence Tomography. He suffered from a cerebral aneurysm in 2010, with subsequent development of secondary hydrocephalus that required surgical management with a ventriculoperitoneal shunt. The patient also mentioned having family members with hypertension. His best-corrected visual acuity was 20/30 OD and 20/25 OS. IOP ranged between 11-12 mmHg in both eyes, on no medication. Corneal pachymetry was 548 microns OD and 543 microns OS. Gonioscopy showed open angles in both eyes.
Slit-lamp examination was unremarkable, and optic nerve evaluation showed cup-to-disc ratios of 0.8 OD and 0.6 OS (Figure 6A and 6B). The patient brought to the consultation an SD-OCT that evidenced thinning of the RNFL in both eyes, and GCL thinning predominantly in the right eye (Figure 6C and 6D). He had an MRI that evidenced severe changes due to his previous neurological history, and Humphrey's visual field revealed left homonymous hemianopia that respects the vertical meridian (Figure 6E and 6F). These findings were indicative of trans neuronal retrograde degeneration due to his previous cerebral aneurysm and hydrocephalus (Figure 7).
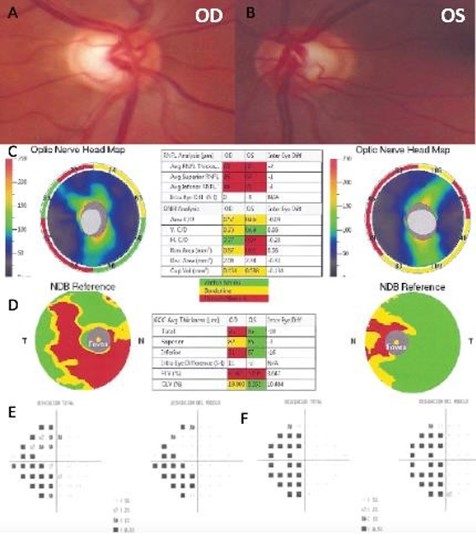
Figure 6. Color fundus photography of the right eye (A) and left eye (B). The spectral-domain optical coherence tomography (SD-OCT) with Optic Nerve Head map (C) and Ganglion Cell Complex analysis (D). Humphrey visual field of the right eye (E) and left eye (F).
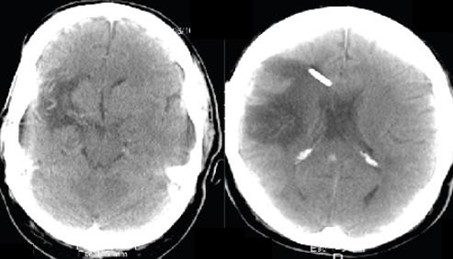
Figure 7. MRI evidencing severe morphological changes and a ventriculo-peritoneal shunt.
Case 4:
In March 2019, a 44-year-old Hispanic male diagnosed with glaucoma came to our center looking for a second opinion after two ophthalmologists suggested a surgical procedure due to elevated IOP and cupping of the optic nerves. His medical history included a Pituitary Adenoma that was resected 20 years ago. Additionally, he referred first-degree family members with hypertension and gastric cancer. His best-corrected visual acuity was 20/20 in both eyes. IOP ranged between 10-11 mmHg in both eyes on Latanoprost. Corneal pachymetry was 556 microns (OD) and 525 microns (OS). Gonioscopy showed open angles in both eyes. Slit-lamp examination was unremarkable, and optic nerve evaluation showed pallor of the neuroretinal rim and cup-to-disc ratios of 0.6 and 0.7 OD and OS, respectively (Figure 8A and 8B).
The patient brought to the consultation an SD-OCT that evidenced thinning of the RNFL predominantly in the superior and temporal quadrant of both eyes. Macular Ganglion Cell Layer thickness was normal (Figure 8C and 8D). Humphrey's visual field evidenced an inferior paracentral defect in the right eye and no remarkable defects in the left eye (Figure 8E and 8F). We suggested an evaluation from a neurosurgeon. In May 2019, the patient returned to our clinic with an MRI that evidenced a residual Parasellar tumor. The neurosurgeon suggested that the lesion has remained stable over the years and did not require any further surgical management (Figure 9). Therefore, we suspended pharmacological therapy, IOP remained stable in the low teens, so it was suggested that he remained under observation without any ocular surgical intervention.
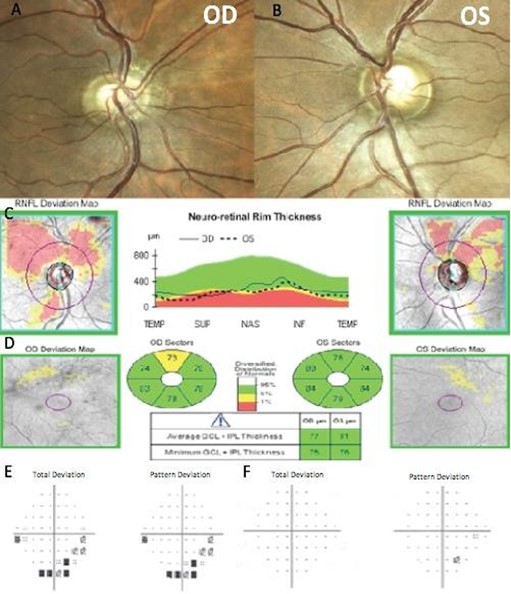
Figure 8. Color Fundus photography of the right eye (A) and left eye (B). The spectral-domain optical coherence tomography (SD-OCT) peripapillary retinal nerve fiber layer thickness analysis (C), and ganglion cell macular thickness analysis (D). Humphrey visual field of the right eye (E) and left eye (F).
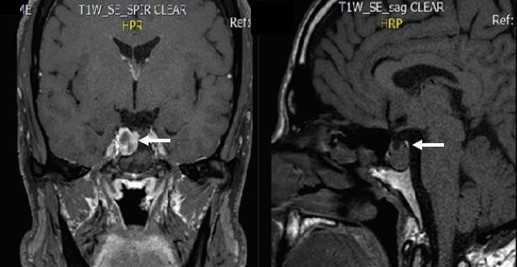
Figure 9. Axial and sagittal cuts of an MRI evidencing a residual parasellar tumor (white arrows).
A summary of the main findings is identified in the case series in Table 1.
Table 1. Case Reports main findings.
|
Case Series |
Case 1 |
Case 2 |
Case 3 |
Case 4 |
|
Gender |
Male |
Male |
Male |
Male |
|
Age |
55 |
38 |
55 |
44 |
|
BCVA |
20/20 OD and 20/100 OS |
20/20 on both eyes |
20/30 OD and 20/25 OS |
20/20 on both eyes |
|
C/D ratio |
0.4 OD, 0.6 OS |
0.5 OD, 0.6 OS |
0.8 OD, 0.6 OS |
0.6 OD, 0.7 OS |
|
IOP |
19-24 mmHg on both eyes |
13-14 mmHg on both eyes |
11-12 mmHg on both eyes |
10-11 mmHg on both eyes, on Latanoprost |
|
Pallor of the optic nerve |
- |
- |
- |
+ |
|
Main OCT Findings |
RNFL thickness 87µm OD, 80µm OS. GCL thickness 62µm OD, 58µm OS |
RNFL thickness 70µm OD, 72µm OS. GCL thickness 61 µm on both eyes |
RNFL thickness 66µm OD, 68µm OS. GCL thickness 76µm OD, 86µm OS |
RNFL thickness 59µm OD, 78µm OS. GCL thickness 77µm OD, 81µm OS |
|
Humphreys visual field |
Temporal hemianopia OS |
Inferior-nasal cuadrantanopsy OD and a temporal defect that respects the vertical line OS |
Left homonymous hemianopia that respects the vertical meridian |
Inferior paracentral defects OD |
|
Diagnosis |
Tuberculum Sellae Meningioma |
Cavernous Hemangioma with a sellar extension |
Trans neuronal retrograde degeneration due to his previous neurological history |
Residual Parasellar tumor |
BCVA: Best Corrected Visual Acuity; OD: Right eye; OS: Left eye; C/D ratio: Cup/Disc ratio; IOP: Intraocular Pressure; OCT: Optical Coherence Tomography: µm: Microns.
Discussion
Our report seeks to emphasize the value of clinical examination and adequate interpretation of diagnostic tools in cases where glaucomatous optic neuropathy is suspected. Recognizing that an incorrect diagnosis of neuroophthalmological pathologies may negatively impact the patient’s quality of life [3,4]. The American Academy of Ophthalmology and different authors propose that some clinical clues may help identify patients who require further comprehensive neuroophthalmological evaluation. [4-6] We aim to highlight how a thorough clinical evaluation and interpretation of OCT and visual fields may help differentiate neurological lesions compromising the visual pathway from glaucoma.
In the first case, identification of diminished visual acuity of the left eye without any remarkable findings on slit-lamp examination, and the presence of an afferent pupillary defect in the left eye could suggest a neuroophthalmological disease. Additionally, the OCT revealed GCL thinning in all sectors, and Humphrey's visual field revealed temporal hemianopia on the left eye. In the second case, identification of visual field loss that respected the vertical meridian, absent disc hemorrhages, and thinning of the RNFL and GCL predominantly in the superior, inferior, and temporal quadrant were suggestive of a pathology different from glaucoma. Our diagnostic suspicion of a neurological condition that compromised the visual pathway was confirmed by neuroimaging brought in subsequent consultations.
Different authors suggest that patients presenting clinical clues such as patients under 65 years of age that have visual field defects; visual field loss that respects the vertical midline; poor disc/field correlation; the pallor of the neuroretinal rim; asymmetrical loss of color vision; relative afferent pupillary defect or absent disc hemorrhages, could benefit from adequate OCT and visual fields interpretation and neuroimaging [4-6]. Ahmed et al. suggested that neuroimaging glaucoma suspect patients could be cost-effective considering the consequences of a late or missed diagnosis of an intracranial lesion affecting the optic chiasm [7].
The third case was referred to as a glaucoma suspect due to thinning of the RNFL and GCL in the Optical Coherence Tomography. We identified that the changes in the OCT and optic disc cupping were related to his previous neurological history evidenced in the MRI. His visual field revealed left homonymous hemianopia which respected the vertical meridian indicative of trans neuronal retrograde degeneration due to his neurological condition. Regarding the fourth case presented, the patient sought a second opinion after a surgical procedure had been advised to control IOP. The patient shared that he had a Pituitary Adenoma resected 20 years before. We suspected that the cupping of his optic nerves, the OCT findings, and Humphrey visual field suggested a residual parasellar tumor, later evidenced on the MRI. We suggested an evaluation by a neurosurgeon, who later confirmed that the lesion had remained stable over the years and did not require any further surgical management. The patient did not require any surgery for glaucoma control, instead, there was a switch in pharmacological therapy to Brimonidine and periodic observation was suggested.
Tumors or space-occupying lesions (aneurysms) located relatively close to the optic nerve may block the free passage of cerebrospinal fluid into the optic nerve canal and the orbit [8]. Different studies suggest a high tendency of parasellar tumors extending into the optic nerve canal [9,10]. Compression of the axons of the anterior visual pathway may result in axonal degeneration, demyelination, and impaired conduction, which primarily affects visual fields and visual acuity [11]. Trans neuronal retrograde degeneration of retinal ganglion cells leads to significant RNFL and RGC thinning, which were present in our patients [12]. Whereas typical glaucomatous RNFL thinning affects the inferior and temporal quadrant predominantly [1,2].
Retinal Nerve Fiber Layer thinning can eventually lead to the loss of Retinal Ganglion Cells and permanent damage of nerve fibers unless timely decompression is done [11,12]. In our first case, after the Tuberculum Sellae Meningioma was surgically removed, the patient recovered visual acuity and visual field of the left eye. In this case, RNFL first measurement of the left eye was 80 microns. Costello et al. found a threshold of RNFL thickness values less than 75 microns to predict incomplete visual field recovery [13]. This finding suggests that OCT has a predictive role so that the degree of reversibility is related to the preoperative RNFL thickness [13]. Visual fields’ recovery could be related to an initial phase of reversible thinning where the impaired visual function may be due to reversible causes such as impaired axon conduction or axoplasmic stasis [14]. Full recovery should be possible once the compression is relieved before permanent atrophy occurs [14].
Pasol et al. suggested that Optical Coherence Tomography should be used concomitantly with the ocular examination when evaluating patients who may have non-glaucomatous cupping of the optic nerve, with attention to central vision, color vision, and visual field defects that are not typical of glaucomatous loss [3]. Prolonged duration of symptoms, poor visual acuity, optic disc pallor, and incomplete tumor resection may serve as risk factors for poor visual outcomes after decompression surgery [14]. Atkinson et al., found considerable remission rates after cranial tumors resections, so rigorous follow-up of these patients should be considered [15].
In conclusion, adequate examination and interpretation of diagnostic tools may help differentiate neurological lesions compromising the visual pathway from glaucoma and prevent scenarios of late diagnosis of treatable intracranial tumors or unnecessary glaucoma treatment. Intracranial compressive lesions must be carefully considered in the workup of patients with glaucomatous appearance of the optic nerve, without a typical correlation of structure-function tests such as OCT and visual fields.
List of Abbreviations:
SD-OCT: Spectral-Domain Optical Coherence Tomography; RNFL: Retinal Nerve Fiber Layer; GCL: Ganglion Cell Layer; IOP:
Intraocular Pressure; OD: Right eye; OI: Left eye; MRI: Magnetic Resonance Imaging.
Declarations:
Ethics approval and consent to participate: The report was carefully developed following the ethical guidelines contemplated in the Declaration of Helsinki and Resolution 8430 of 1993 of the Ministry of Health, and in accordance with this resolution was considered a risk-free study.
Consent for publication: Written informed consent for publication of the clinical details and clinical images was obtained from the patients. A copy of the consent form is available from the corresponding author on reasonable request.
Availability of data and material: Available from the corresponding author on reasonable request.
Competing interests: The authors declare that they have no competing interests.
Funding: The authors declare that they have not received any kind of funding for the realization of this paper.
Authors' contributions: SBR conceived the need to write up the cases. MEME, JED, and AMRS did the literature search and write-up of the manuscript. All authors discussed and contributed to the final version of the manuscript.
References- Mushtaq S, Munir N. Role of Ocular Coherence Tomography in Glaucoma Diagnosis. Ophthalmology Update. 2017;15(2):55-58.
- Hwang YH, Kim Y, Chung JK, Lee KB (2018) Glaucomatous progression in the retinal nerve fibre and retinal ganglion cell- inner plexiform layers determined using optical coherence tomography-guided progression analysis. Clinical and Experimental Optometry. 101(5): 666-73.
- Pasol J (2011) Neuro-ophthalmic disease and optical coherence tomography: glaucoma look-alikes. Current Opinion in Ophthalmology. 22(2): 124-32.
- Dias DT, Ushida M, Battistella R, Dorairaj S, Prata TS (2017) Neurophthalmological conditions mimicking glaucomatous optic neuropathy: analysis of the most common causes of misdiagnosis. BMC Ophthalmology. 17(1).
- Stein JD, Challa P (2007) Diagnosis and Treatment of Normal Tension Glaucoma. American Academy of Ophthalmology [Internet].
- Perera N, Shields M, Perera M, Adler PA (2019) When 'glaucomatous fields' are not glaucoma: bilateral calcarine fissure strokes masquerading as glaucoma in a normal tension glaucoma suspect. BMJ Case Reports. 12(3): e227803.
- Ahmed IIK, Feldman F, Kucharczyk W, Trope GE (2002) Neuroradiologic screening in normal-pressure glaucoma: Study results and literature review. Journal of Glaucoma. 11(4): 279-86.
- Qu Y, Wang YY, Xu L, Zhang L, Zhang J, et al. (2011) Glaucoma-like optic neuropathy in patients with intracranial tumours. Acta Ophthalmologica. 89(5): e428-e433.
- Mahmoud M, Nader R, Al-Mefty O (2010) Optic canal involvement in tuberculum sellae meningiomas: Influence on approach, recurrence, and visual recovery. Neurosurgery. 67(3 Suppl Operative): 108-18.
- Sade B, Lee JH (2009) High incidence of optic canal involvement in tuberculum sellae meningiomas: rationale for aggressive skull base approach. Surgical Neurology. 72(2): 118-23.
- Park HH, Oh MC, Kim EH, Kim CY, Kim SH, et al. (2015) Use of optical coherence tomography to predict visual outcome in parachiasmal meningioma. J Neurosurg. 123(6):1489-99.
- Monteiro MLR, Hokazono K, Fernandes DB, Costa-Cunha LVF, Sousa RM, et al. (2014) Evaluation of Inner Retinal Layers in Eyes With Temporal Hemianopic Visual Loss From Chiasmal Compression Using Optical Coherence Tomography. Investigative Ophthalmology & Visual Science. 55(5): 3328- 3336.
- Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, et al. (2006) Quantifying axonal loss after optic neuritis with optical coherence tomography. Annals Of Neurology. 59(6): 963-69.
- Loo J-L, Tian J, Miller NR, Subramanian PS (2013) Use of optical coherence tomography in predicting post-treatment visual outcome in anterior visual pathway meningiomas. British Journal of Ophthalmology. 97(11): 1455-58.
- Atkinson AB, Kennedy A, Wiggam MI, McCance DR, Sheridan B (2005) Long-term remission rates after pituitary surgery for Cushing's disease: the need for long-term surveillance. Clinical Endocrinology. 63(5): 549-59.



