Adama Mawulikplimi Ephoevi-Ga1*, Léhleng Agba2, Mawuli Komi Santos3, Komla Nyinèvi Anayo1, Bitankadé Kabassem1, Claire Assima4, Kokou Mensah Guinhouya4, Kossivi Apetse1, Komi Assogba4, Vinyo Kumako2, Damelan Kombate5, Koko Roger Kuaovi6, Kokou Vonor7, Mofou Belo4, Agnon Koffi Balogou1
1Department of Neurology, CHU campus (Lomé, Togo).
2Department of Neurology, CHU Kara (Kara, Togo)
3Department of Ophthalmology, University of Lomé (Lomé, Togo)
4Department of Neurology, CHU SO (Lomé, Togo).
5Department of Neurology, CHR Kara (Kara, Togo)
6AFIA ophthalmology practice (Lomé, Togo).
7Department of Ophthalmology, University of Kara (Kara, Togo).
*Corresponding Author: Adama Mawulikplimi EPHOEVI-GA, Department of Neurology, University Hospital of campus, Lomé, Togo.
Abstract
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system. We report two MS cases in Togo.
The first patient was 54 years old, hypertensive and diabetic, admitted with right hemiparesis and left VI paralysis. A stroke was suspected, and the etiological work-up revealed diagnostic problems. The diagnosis of MS was accepted. He received background treatment with interferon beta-1a. Progression remained stationary with an EDSS score of 1.5. The second was a 17-year-old adolescent with a history of regressive right upper limb deficit, optic neuropathy, gait and balance problems, bilateral statokinetic cerebellar syndrome, bilateral pyramidal syndrome, right sensory syndrome, and sphincter disorders. He received background treatment with azathioprine. Progression remained stable, with an EDSS score of 6.0.
Keywords: multiple sclerosis, black subject, Togo.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS). It is the most frequent cause of non-traumatic disability in young people [1]. The etiological factors of MS are multifactorial, both genetic and environmental [2]. Epidemiological data on MS are heterogeneous worldwide [3]. Very high prevalence has been described in North America and Europe, while lower prevalence has been reported in East Asia and sub- Saharan Africa [4]. The diagnosis of MS is currently based on the McDonald's criteria [5]. MRI is vital in this diagnostic process, based on the Barkhof criteria [6].
We report two MS cases: a Togolese-Senegalese patient who lived in France and a Togolese who lived in Gabon.
Case No. 1:
A 54-year-old black, right-handed patient of French nationality of Togolese-Senegalese origin, who had lived in France since age 20, had been living in Togo since 2001 for professional reasons. In January 2012, he suddenly presented with horizontal diplopia and, the following day, heaviness of the right upper limb. His cardiovascular risk factors included high blood pressure, type 2 diabetes, being overweight, and active smoking at 20 pack years. Examination revealed a body mass index (BMI) of 29, the overall integrity of upper functions, right hemiparesis rated 4/5 with craniofacial predominance, and paralysis of the left sixth pair. An emergency brain scan was normal.
Brainstem infarction is evoked by the sudden onset and clinical alternating brainstem syndrome. Cerebral arterial and supra-aortic angioscanner, biological work-up, and electrocardiogram were normal. Acetylsalicylic acid 100 mg was introduced, cerebro- cardiovascular risk factors were treated, and the patient benefited from motor physiotherapy sessions.
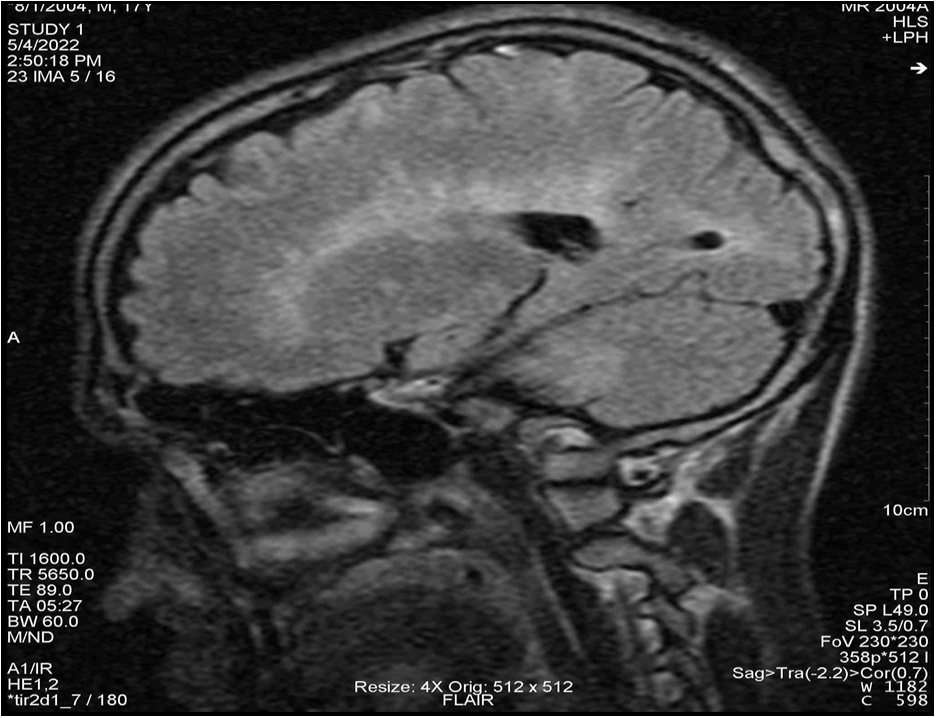
At the patient's request, he continued etiological explorations at a hospital in France in March 2012. Spinal cord and brain MRI revealed an ovoid lesion with T2 hypersignal measuring 10 mm at the level of the vertebral body of C5 and several intra-axial nodular lesions measuring more than 3 mm with T2 hypersignal and T1 iso-signal in the supra- and infra-tentorial white matter (Figure 1). After the injection of gadolinium, enhancement of the lesion was noted in the elongated medulla and was consistent with albumin-cytological dissociation, with hyperproteinorachia at 0.8 g/l and intrathecal IgG synthesis with oligoclonal bands. Somatosensory evoked potentials were slowed in the lower limbs. A relapse of MS was diagnosed, and 1 g of methylprednisolone daily for 3 days was administered, with complete regression of ocular symptoms. At the 3-month follow-up, there was no temporal, clinical, or radiological dissemination, so no background treatment was instituted.
In September 2012, the patient suddenly manifested unilateral orbital pain with an impression of decreased bilateral visual acuity, electric discharge-type paresthesia in the lower limbs, and memory problems predominantly involving anterograde memory, all in the context of a flu-like illness. A brain MRI in December 2012 revealed a subcortical lesion in the left precentral gyrus with fine enhancement in favor of temporal dissemination. Spatial dissemination criteria were also present. Visual evoked potentials showed pathological prolongation of P100 wave latency on the right and left, and somesthetic evoked potentials showed abolition of the right and left medial popliteal sciatica, so multimodal evoked potentials favored MS.
The diagnosis of relapsing-remitting MS was finally accepted, and background treatment with interferon beta-1a was started. Progression remained stationary with an EDSS score of 1.5.
Figure 1: Brain MRI in FLAIR and T2 sequences showing numerous ovoid periventricular hypersignals.
Case No. 2:
A 17-year-old black, right-handed teenager from Togo arrived in Lomé in August 2021 to study at university.
His personal history was mainly atopic. The symptoms began in Libreville, where he lived with his parents. In March 2021, he suddenly presented with right hemicorporeal heaviness with brachial predominance, followed 2 weeks later by visual blur. In August 2021, in Lomé, ophthalmologists diagnosed retrobulbar optic neuritis. Brain and optic tract MRI in September 2021 had returned normal. Biological tests were unremarkable. In January 2022, he developed balance problems.
It will be sent to us at the beginning of March 2022. He had been reporting urinary urgency for a week. Clinical examination revealed a weight of 55 kg with a BMI of 20, good general condition, normal blood pressure, and preserved upper limb function. The pupils were of average size and reactive, and there was no nystagmus. There was distal paresis of the right upper limb at 4/5. Osteotendinous reflexes were vivid in all 4 limbs, polykinetic, and diffused with an epileptoid tremor of the feet. The cutaneous-plantar reflex was in bilateral extension. There was algesic, tactile hyperesthesia of the right upper limb, hypoaesthesia of the right hemicorpus, a statokinetic cerebellar syndrome, and bilateral proprioceptive ataxia. The EDSS score was 4.0. He was seen again a month later, and his balance had deteriorated. He walked with an English cane, complained of worsening micturition impediment, and had very marked static and kinetic cerebellar ataxia.
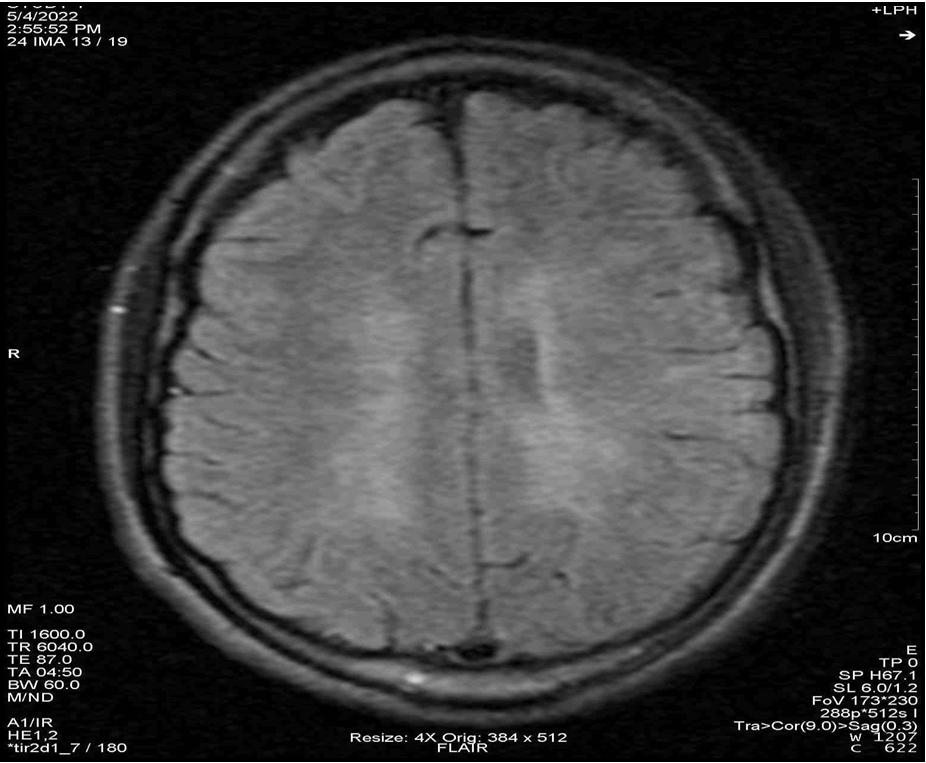
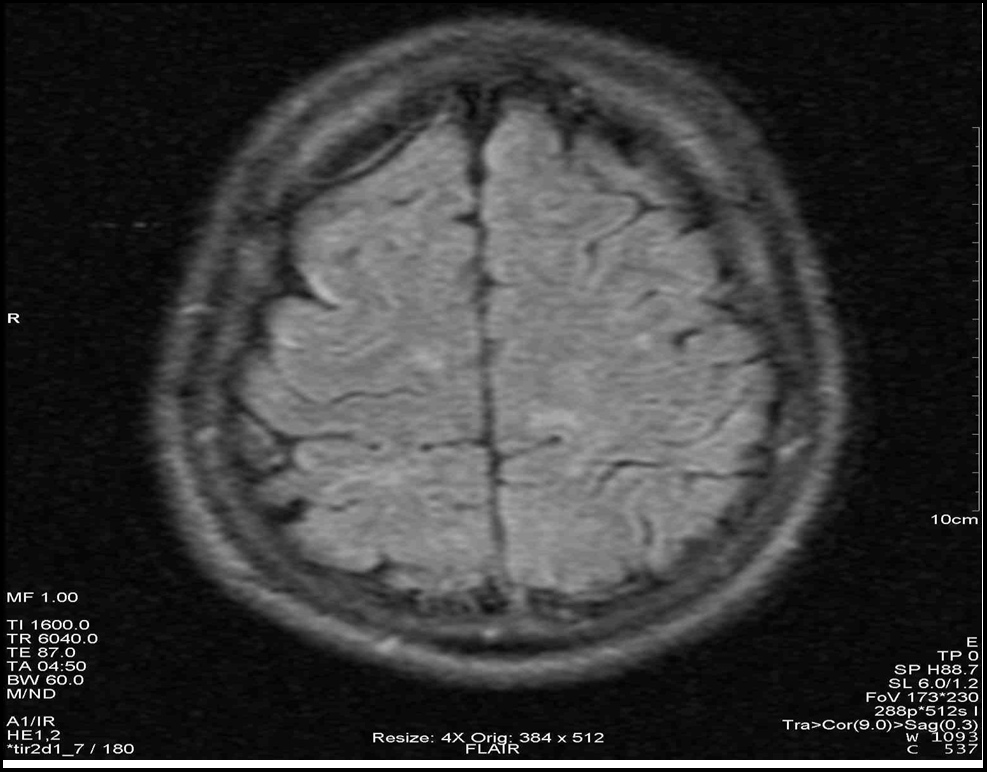
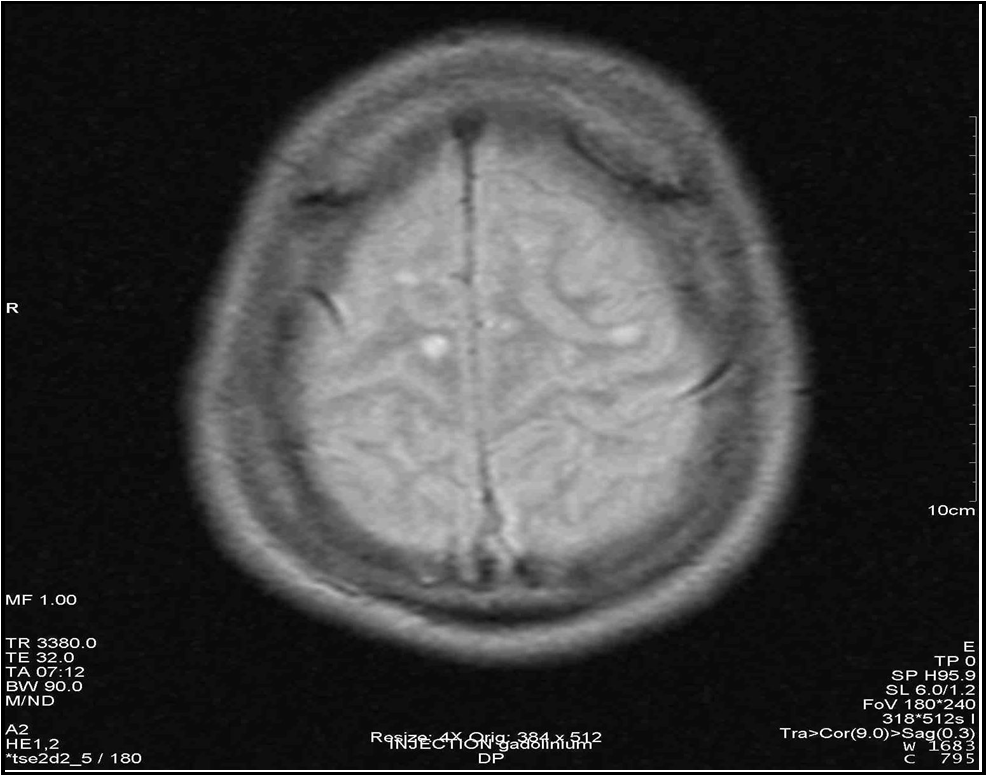
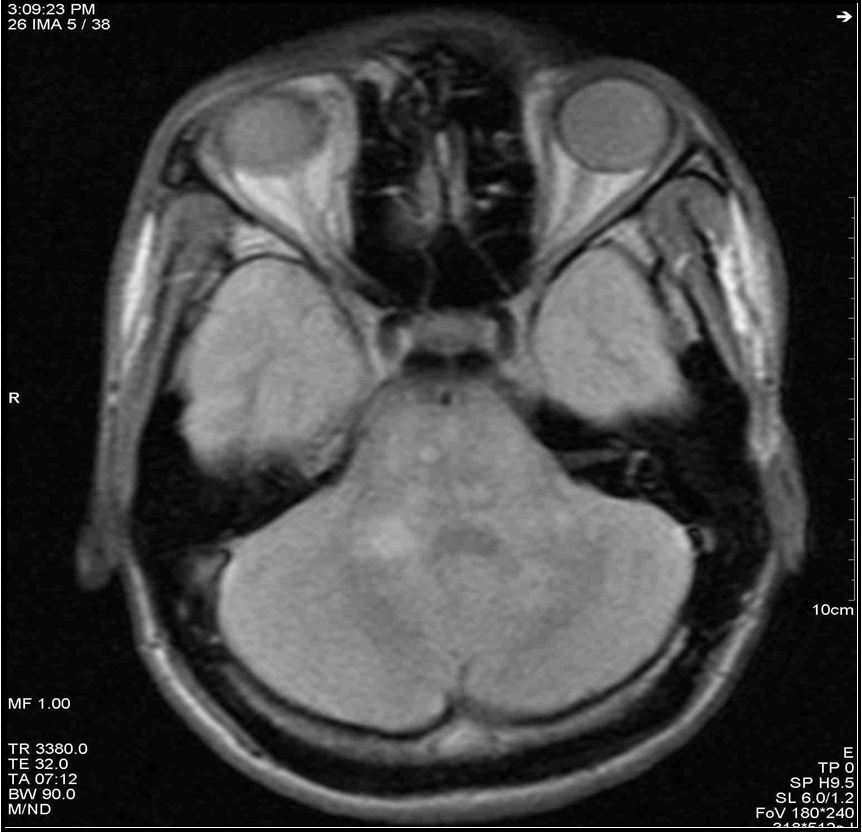
Given this clinical picture, which was highly suggestive of MS, brain and spinal cord MRI at this stage revealed multiple periventricular, corpus callosum, brainstem and right superior cerebellar peduncle hypersignals, an extensive C4 to C7 cervical cord hypersignal, gadolinium contrast of a right parietal hypersignal and of the right superior cerebellar peduncle (Figures 2 to 7). It should be noted that the spinal cord MRI was of questionable quality.
CSF analysis revealed a proteinopathy of 0.59 g/l. LCS isofocalization performed in France revealed oligoclonal bands. Tests for anti-aquaporin 4 and anti-myelin oligodendrocyte glycoprotein antibodies were negative.
It was concluded to be an MS relapse, and 1 g/day of methylprednisolone was given as an IV bolus for five days. One week later, his motor balance and bladder and bowel function had improved. The diagnosis of relapsing-remitting multiple sclerosis was accepted, and a disease-modifying treatment with azathioprine 50 mg: 1 tablet x3/day was introduced in June 2022. He benefits from physiotherapy sessions. Progression remained stable, with an EDSS score of 6.0.
Figure 2: Sagittal section in FLAIR sequence showing hypersignals of the corpus callosum, thalamus and cerebellum.
Figure 3: Sagittal section in FLAIR sequence showing periventricular hypersignals.
Figure 4: Axial section in FLAIR sequence showing several parietal hypersignals.
Figure 5: Axial section in FLAIR sequence showing enhancement of a right parietal hypersignal.
Figure 6: Axial section in FLAIR sequence showing hypersignal of the right upper cerebellar peduncle and hypersignals of the brainstem.
Figure 7: Axial section in T2 sequence showing hypersignal enhancement of the right superior cerebellar peduncle.
Discussion
Both observations posed diagnostic problems. The first patient was 54 years old at the time of diagnosis. Given the sudden onset of the disease, a stroke was suspected, but after non-contributory investigations, the diagnosis of MS was finally made in France. McDonald's diagnostic criteria [5] were used to retain the diagnosis of MS in both patients.
The second observation is characterized by a severe neurological picture with relapses every few days or weeks. This is why it was not easy to distinguish the form of MS in this adolescent. Extreme forms of MS have been described as benign and malignant forms. The frequency of mild forms is estimated to be between 5% and 40%. Gentle evolution correlates with low initial disease activity on MRI, i.e., fewer new lesions [7].
Malignant forms (fulminant, aggressive, explosive onset) of MS correspond to forms of the disease characterized clinically either by an inaugural attack with severe clinical expression or by the existence of a rapidly aggressive course represented by frequent and severe attacks with sequelae or the constitution of a deficit which progresses constantly during the first months of the course. The radiological correlate of these forms with a powerful presentation is usually represented on MRI by the presence on the initial examination of numerous lesions, sometimes large ("tumoral" or "pseudotumoral" lesions over 2 cm in diameter), and significant inflammatory activity, represented by the existence of numerous gadolinium uptakes [8].
This teenager had numerous lesions on MRI and at least two gadolinium contrast images. This observation suggests that an aggressive form of MS could be seen in a black patient who has never left Africa. These antagonistic forms can cause diagnostic problems even in countries like France, where in 2010, Brochet et al. reported 2 cases with diagnostic difficulties [9].
In this second patient, the diagnosis of MS was made 14 months after the first neurological event. Several factors could explain this delay in diagnosis: low incidence, lack of awareness of the disease, and poor availability of diagnostic tests.
Firstly, the incidence is low in sub-Saharan Africa. 2014 Okubadejo et al. [10] reported in Lagos (Nigeria), from a total of 3960 patients over five years, 5 cases of clinically defined MS, and an incidence of 1 case/year. In 2016, Assadeck et al. [11] reported the first 2 cases in Niger in 20 years of neurology activity. In 2001, in Nairobi, Kenya, Kioy et al. [12] found a similar incidence, with 9 cases of clinically defined MS out of 2,831 patients seen over 10 years.
Secondly, nursing staff and patients' lack of awareness of the disease could explain this delay. It is not specific to Black Africa. Basraoui et al. [13] in Morocco, comparing the diagnostic time between the first attack and the first medical consultation, found a period of 36 months with an average EDSS of 2.9. The first MRI and 1st lumbar puncture were performed after 36.3 and 40.8 months respectively. The diagnosis was confirmed after 46.4 months, and treatment was initiated after 47.8 months. They explained this delay by the downplaying of symptoms, the difficulty of accessing care, and the Moroccan population's lack of awareness of the initial signs.
Lastly, this delay could be explained by the limited availability of diagnostic testing resources in Black Africa. Grunitzky et al. [14] 2001 already explained the rarity of MS by the absence and modesty of neurological infrastructures. The 2017 McDonald's diagnostic criteria [5] involve the performance of encephalic and spinal cord MRI and the search for oligo-clonal bands on LCS isoelectrofocusing. According to France's Haute autorité de santé, MRI must be performed on a machine of at least 1.0 Tesla and include at least the following sequences: T1, FLAIR, T2 double echo, and T1 performed 5 minutes after injection of a single dose of gadolinium [15]. In a low- income country where universal access to healthcare is not yet operational, it is often difficult to renew a request for an MRI scan to confirm the diagnosis or for a follow-up of this condition.
The first patient posed no therapeutic problems, as he was covered by a French health insurance plan with an exemption from co-payment. Treatment of the relapse was carried out, and background treatment with interferon beta-1a was introduced.
As for the second patient, it was clear that there would be a therapeutic problem. The flare-up was treated with an IV bolus of corticosteroids. In active forms, high-dose methylprednisolone (5-10 g) has been proposed; if this fails, plasma exchange should be considered [8], [16]. A dose of 1 g of methylprednisolone was administered to our patient, and background treatment was introduced.
Early initiation of disease-modifying therapy is recommended in MS to reduce the frequency of relapses and the progression of short-term disability [15]. In France, four immunosuppressive drugs are indicated for very active relapsing-remitting MS with or without progression of disability (natalizumab, fingolimod, ocrelizumab, and mitoxantrone), but in the vast majority of cases in Black Africa, azathioprine remains the only therapeutic alternative. Studies on azathioprine are too old to meet the required evidence level. However, they suggest an efficacy on the number of relapses, with no effect on the evolution of disability [15]. We do not yet have enough experience to assess the effectiveness of this therapy.
Conclusion
MS in sub-Saharan Africa remains under-diagnosed. The low incidence is explained by a need for more awareness of the pathology and inadequate technical facilities. A doctor's primary concern with any retrobulbar optic neuritis should be to perform a cerebral MRI and a lumbar puncture.
Declarations
Consent for publication : Written informed consent has been obtained from all patients to publish the case details.
Competing Interests: The authors declare that they have no competing interests.
Funding: No funding to report.
References
- Goodin DS (2014) The epidemiology of multiple sclerosis: insights to disease pathogenesis. Handb Clin Neurol. 122: 231– 66.
- Confavreux C, Vukusic S (2006) Natural history of multiple sclerosis : a unifying concept. Brain. 129(pt 3): 606–616.
- Federation MSI. Atlas of MS 2013: mapping multiple sclerosis around the world; 2013. Accessed 17 Oct 2022.
- Leray E, Moreau T, Fromont A, Edan G (2016) Epidemiology of multiple sclerosis. Rev Neurol (Paris). 172(1): 3-13.
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, et al. (2018) Diagnosis of multiple sclerosis : 2017 revisions of the McDonald criteria. Lancet Neurol. 17(2): 162‑173.
- Myhr KM (2008) Diagnosis and treatment of multiple sclerosis. Acta Neurol Scand. 188: 12–21.
- Kidd D, Thompson AJ, Kendall BE, Miller DH, McDonald WI (1994) Benign form of multiple sclerosis: MRI evidence for less frequent and less inflammatory disease activity. J Neurol Neurosurg Psychiatry. 57(9): 1070–1072.
- Lucchinetti CF, Gavrilova RH, Metz I, Parisi JE, Scheithauer BW, et al. (2008) Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 131(7): 1759‑1775.
- Brochet B, Clavelou P, Moreau T, Pelletier J (2010 ) Sclérose en plaques à début sévère. Prat. Neurol – FMC. 1(1): 7‑14.
- Okubadejo N, Ojo O, Lawal T, Ojini F, Ma D (2014) Unveiling Multiple Sclerosis in Nigeria: The conundrum of diagnosis and access to disease modifying therapies. Neurology. 82: 51-52.
- Assadeck H, Daouda MT, Djibo FH, Maiga DD, Omar EA (2018) Premiers cas de sclérose en plaques au Niger. Bull Soc Pathol Exot. 111: 77-80.
- Kioy PG (2001) Emerging picture of multiple sclerosis in Kenya. East Afr Med J. 78(2): 93–6.
- Basraoui S, Naliri D, Khattab H, Bellakhdar S, El Otmani H, et al. (2020) La sclérose en plaques : pathologie méconnue par les marocains ? Impact du symptôme inaugural et de l’âge de début sur le délai de diagnostic. Rev. Neurol. 176: 124‑125.
- Grunitzky EK, Balogou AAK, Kowou AL (2001) La sclérose en plaques en Afrique noire. AJNS. 20(1): 4–6.
- https://www.has-sante.fr/jcms/c_272001/fr/la-sclerose-en- plaques.
- Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, et al. (2002) Plasma exchange for severe attacks of CNS demyelination:predictors of response. Neurology. 58(1): 143-6.