Khadija Laasri*, Salma Marrakchi, Zakia El Yousif, Ittimade Nassar, Nabil Moatassim Billah
Radiology department. Ibn Sina University Hospital, Mohammed V University, Rabat, Morocco.
*Corresponding Author: Khadija Laasri, Radiology department. Ibn Sina University Hospital, Mohammed V University, Rabat, Morocco.
Abstract
Neurofibromatosis type 2 is an inherited autosomal dominant syndrome characterized by multiple neoplasms of the central and peripheral nervous system associated with ocular abnormalities. The most prevalent tumor related to the disease is the vestibulocochlear schwannoma (VIII cranial nerve), and as many as 10% of patients with this tumor have neurofibromatosis type 2. This report aims to present a 34-year-old male who was seen for bilateral hearing loss. During his workup, which included cranial computer tomography, he was found to have multiple intracranial masses. Cranial and whole spine magnetic resonance imaging revealed bilateral vestibulocochlear schwannoma, various meningiomas, and one intramedullary tumor. Based on clinical and imaging findings, the diagnosis of neurofibromatosis type 2 was made.
Keywords: Neurofibromatosis type 2, tumor, MRI, schwannoma - meningioma - ependymoma
Introduction
Neurofibromatosis type 2 is a rare autosomal dominant disorder, with an incidence of 1 in 33,000 to 40,000 [1,2]. Male and female patients are affected approximately equally [3]. Until 1987, neurofibromatosis types 1 and 2 were considered the same disease, but research has proven that the two syndromes are caused by distinct mutations in various chromosomes [4]. The disease is caused by mutations of the NF2 gene on chromosome 22q12 [5]. Approximately half of the cases are familial (inherited), while the other half occur due to de novo (sporadic) mutations [6]. The purpose of this report is to provide a case of sporadic NF2, whose diagnosis was made based on a patient's medical history, clinical symptoms, and magnetic resonance imaging (MR) results.
Case Report
We present the case of a 24-year-old man with no prior surgical, medical, or familial history, which was seen at an outside facility for progressive bilateral hearing loss. A head computed tomography (CT) showed multiple intracranial masses. He was then referred to our institution for further workup. Physical examination revealed an alert and oriented patient with normal cognitive function. He had bilateral hearing loss and diminished visual acuity. A few scattered skin lesions that resembled skin tags were also found. He was referred for an otologic evaluation, which included an audiometric exam that showed a bilateral sensorineural hearing loss, profound on the left and moderate on the right. Also, the brainstem evoked response audiometry revealed no response on the left and only one wave on the right.
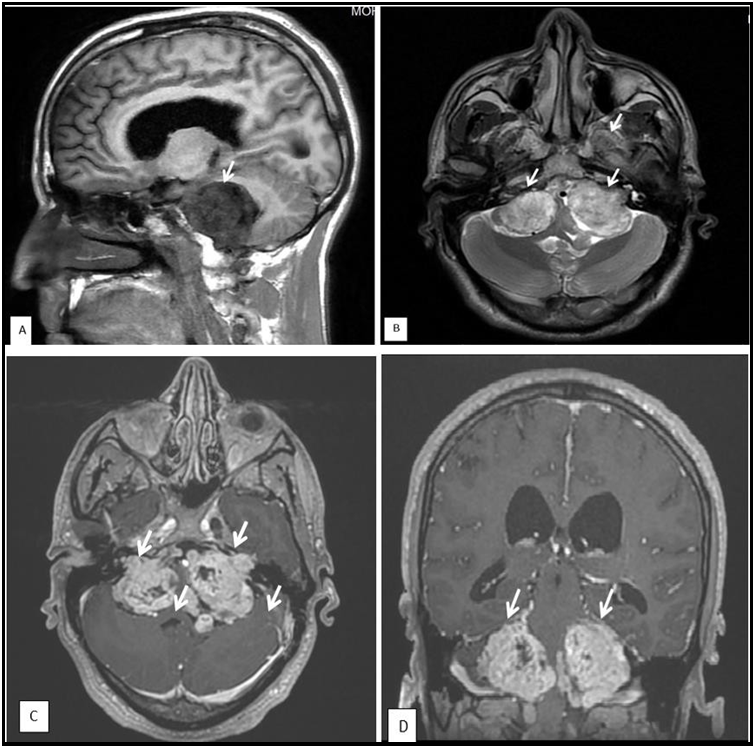
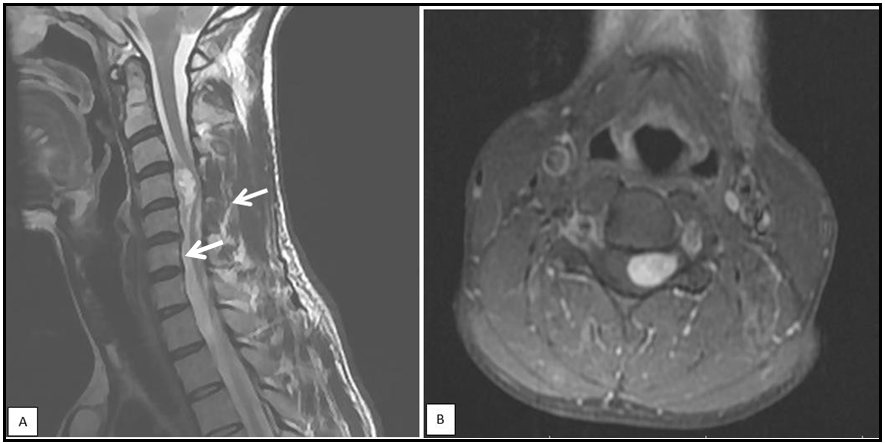
Review of the brain MR imaging findings showed bilateral acoustic schwannoma (figure 1), and another four enhancing masses consistent with meningiomas, involving the parafalcine region, sphenoid wing, and frontal and parietal lobes (Figure 2). Spine MR imaging findings included one cervical intramedullary tumor and one cervical spine meningioma (Figure 3). He was then referred to Neurosurgery Department for surgical treatment. Although he had no family history, the image findings of bilateral acoustic schwannoma, multiple intracranial meningiomas, and one intramedullary tumor suggested the diagnosis of NF2.
Figure 1 : Vestibular schwannomas. Sagital (A) T1- weighted MR images, axial (B) T1- weighted MR images, and axial (C) and coronal (D) enhanced T1-weighted MR images, demonstrating solid bilateral masses in the cerebellopontine angles, compressing the pons and the 4th. ventricle
Figure 2 : Coronal (A) and axial (B) T1 weighted contrast-enhanced MR images showing a dural-based homogeneous enhancing masse, suggesting the diagnosis hypothesis of meningiomas ( arrow).
Figure 3: sagittal T1- weighted MR images (A) and axial T1-weighted contrast-enhanced MR images (B) of the cervical spine showing a mass on hyperintense at the C4 level (arrow), compatible with intramedullary tumor (ependymoma or astrocytoma).
Discussion
The NF 2 syndrome has been known as "MISME" since it is characterized by numerous hereditary schwannomas, meningiomas, and ependymomas (E). Along with the neoplasms, juvenile cortical cataract (posterior subcapsular lenticular opacity) is frequently seen.
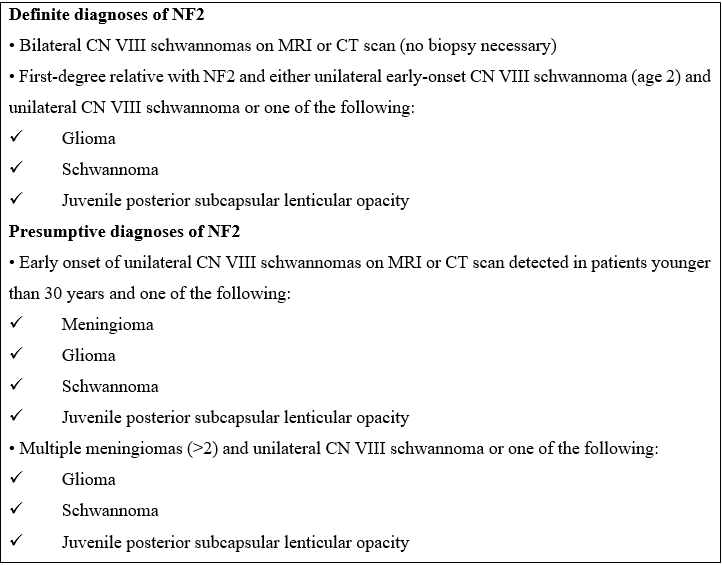
Diagnostic criteria have been developed to allow a clinical diagnosis of the condition even before bilateral vestibular schwannomas have been created. In 1997 Gutmann et al. proposed revised criteria for diagnosing the syndrome [7]. (Table 1).
Table 1. [Gutmann et al (7)]
NF2 is often diagnosed in the second or third decade of life, peaking in the twenties. The clinical manifestation of NF2 varies. However, 30 and 45 % of individuals are diagnosed because of cranial nerve (CN) VIII schwannomas, including hearing loss, tinnitus, balance impairment, and weakening in the CN VII distribution. Direct compression of the cochlear nerve or a diminution in local blood supply is also a potential cause of progressive hearing loss. This is because CN VIII schwannomas exhibit symptoms at a relatively small size. The hearing loss is progressive and caused either through direct compression of the cochlear nerve or reduction of regional blood flow, or the tumor causes hemorrhage into the nerve or cochlea [8]. In the Mautner et al. [9] series, 43 patients (90 %) had bilateral CN VIII schwannomas, while 3 individuals (6 %) had unilateral CN VIII schwannomas.
Several authors have studied a series of issues to determine the frequency of malignancies in NF2 cases. When 48 patients with NF2 were reviewed by Mautner et al., the following common abnormalities were found: vestibular schwannomas (CN VIII) in 46 (96 %), spinal tumors in 43 (90 %), posterior subcapsular cataracts in 30 (63 %), meningiomas in 28 (58 %), and trigeminal schwannomas in 14 (29 %) [9]. The cranial MR of 11 patients was reported by Aoki et al. [10]. The patients in their series included 6 meningiomas, 5 multiple cranial nerve tumors, and 8 additional cranial nerve tumors in addition to acoustic schwannomas (4 multiple and 2 single).
Ependymoma is the most common intramedullary spine tumour in NF2, arising from either the CONUS or the upper cervical cord. Meningiomas typically affect the thoracic spine and manifest as intradural extramedullary neoplasms, similar to incidental meningiomas. They are frequently multiple in number. Petronas et al.'s study of 49 patients with NF2 using spinal MR imaging revealed malignancies in the spinal cord and canal in 31 (63 % of the patients). 22 patients (45 %) had at least one tumour of each category, while 26 patients (53 %) had intramedullary lesions and 27 patients (55 %) had intradural extramedullary tumors [12].
Our patient had bilateral CN VIII schwannomas, four meningiomas, two intracranial and two spinal, and one intramedullary tumour, with no histopathological diagnosis. The schwannomas were the cause of his bilateral hearing loss. Although he had no family history, the finding of bilateral schwannomas is diagnostic for the syndrome without needing a biopsy.
Given their propensity for bilateral VS, patients with NF2 have routine ophthalmologic exams, complete neurologic evaluation for myelopathy, mononeuropathy, and audiology testing [13-14]. The diagnosis and treatment of patients with NF2 have long relied on imaging [15]. Specifically, MR imaging of the CNS is performed at baseline. Internal auditory canal (IAC) axial and coronal thin-section imaging during brain MR imaging should be done to detect vestibular syndrome (VS) [14]. Nearly all NF2 patients should get an annual brain MRI with hin cuts through the IAC, and individuals who are at risk for brainstem compression or impairment of the facial or vestibular nerves sometimes require one every three to six months [16]. Given the propensity for spinal ependymomas, schwannomas, and meningiomas, which are included in the National Institutes of Health (NIH) diagnostic criteria, MR imaging of the spine with contrast should also be performed [14]. The frequency of this imaging depends on the tumour burden and clinical exam findings. Patients with NF2 are continually monitored clinically and by imaging to determine whether the abnormalities described above are stable [17]. Following an initial evaluation, surveillance with contrast-enhanced MR imaging of the brain and IACs (± spine if there are spinal lesions present) is performed in addition to every 6 to 12-month ophthalmological exam and audiology testing [16].
Optimum treatment is multidisciplinary because of the complexities associated with managing the multiple, progressive, and protean lesions related to the disorder [13].
Conclusion
Increased understanding of the clinical manifestations of neuro fi bromate sis type 2 in conjunction with improved neuro fi bromate sis type 2 in conjunction with enhanced precision of genetic tests and imaging studies have accuracy. Genetic tests and imaging studies have improved the early diagnosis of patients. MRI is the gold standard for imaging brain and spinal tumors. Currently, there is no alternative modality with equivalent image quality.
Author’s Contributions: All authors contributed to this work. All authors have read and approved the final version of the manuscript.
Conflicts of Interest: The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- Evans DG, Huson SM, Donnai D, Neary W, Blair V, et al. (1992) A genetic study of type 2 neurofibromatosis in the United Kingdom. I. Prevalence, mutation rate, fitness, and confirmation of maternal transmission effect on severity. J Med Genet. 29(12): 841-6.
- Mautner VF, Tatagiba M, Lindenau M, Fünsterer C, Pulst SM, et al. (1995) Spinal tumors in patients with neurofibromatosis type 2: MR imaging study of frequency, multiplicity, and variety. AJR Am J Roentgenol.165(4): 951-5.
- Baser ME, Evans DG (2000) Lack of sex-ratio distortion in neurofibromatosis 2. Am J Med Genet. 95(3): 292.
- Rouleau GA, Wertelecki W, Haines JL, Hobbs WJ, Trofatter JA, et al. (1987) Genetic linkage of bilateral acoustic neurofibromatosis to a DNA marker on chromosome 22. Nature. 329(6136): 246-8.
- Wippold FJ 2nd, Lubner M, Perrin RJ, Lämmle M, Perry A (2007) Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns. AJNR Am J Neuroradiol. 28(9): 1633-8.
- Antinheimo J, Sankila R, Carpén O, Pukkala E, Sainio M, et al. (2000) Population-based analysis of sporadic and type 2 neurofibromatosis-associated meningiomas and schwannomas. Neurology. 54(1): 71-6.
- Gutmann DH, Aylsworth A, Carey JC, Korf B, Marks J, et al. (1997) The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA.278(1): 51-7.
- Novak MA: Hearing Loss in Neurotologic Diagnosis In: Neurotology. Edited by Jackler RK, Brackman DE. St Louis, Mosby Year Book Inc; 1994:131-44.
- Mautner VF, Lindenau M, Baser ME, Hazim W, Tatagiba M, et al. (1996) The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery. 38(5): 880-5.
- Aoki S, Barkovich AJ, Nishimura K, Kjos BO, Machida T, et al. (1989) Neurofibromatosis types 1 and 2: cranial MR findings. Radiology. 172(2): 527-34.
- Patronas NJ, Courcoutsakis N, Bromley CM, Katzman GL, MacCollin M, et al. (2001) Intramedullary and spinal canal tumors in patients with neurofibromatosis 2: MR imaging findings and correlation with genotype. Radiology. 218(2): 434- 42.
- Spilberg G, Marchiori E, Gasparetto EL, et al. (2009) Magnetic resonance findings of neurofibromatosis type 2: a case report. Cases Journal. 2: 6720.
- Asthagiri AR, Parry DM, Butman JA, Kim HJ, Tsilou ET, et al. (2009) Neurofibromatosis type 2. The Lancet.373(9679): 1974– 86.
- Ahlawat S, Blakeley JO, Langmead S, Belzberg AJ, Fayad LM (2020) Current status and recommendations for imaging in neurofibromatosis type 1, neurofibromatosis type 2, and schwannomatosis. Skeletal radiology.49(2): 199-219.
- Patronas NJ, Courcoutsakis N, Bromley CM, Katzman GL, MacCollin M, et al. (2001) Intramedullary and spinal canal tumors in patients with neurofibromatosis 2: MR imaging findings and correlation with genotype. Radiology. 218(2): 434– 42
- Plotkin SR, Wick A (2018) Neurofibromatosis and schwannomatosis. Semin Neurol. 38(1): 73–85.
- Baser ME, Friedman JM, Aeschliman D, Joe H, Wallace AJ, et al. (2002) Predictors of the risk of mortality in neurofibromatosis 2. Am J Hum Genet.71(4): 715–23.