Sara González Castro1, Ignacio Jara Alonso1, Raquel Morillo Guerrero1,2,3*
1Pneumology Service. Ramón y Cajal University Hospital, Madrid, Spain.
2Ramón y Cajal Institute for Health Research IRYCIS, Madrid, Spain.
3Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), Madrid, Spain.
*Corresponding Author: Raquel Morillo Guerrero, Centro de Investigación Biomédica en Red de Enfermedades Respiratorias (CIBERES), Madrid, Spain.
Introduction
The clinical spectrum of patients infected with SARS-CoV-2 varies from asymptomatic subjects to patients with acute respiratory distress syndrome (ARDS). The initial stage is characterized by unimpressive viral pneumonia, although in some patients the onset of the immune response produces diffuse alveolar damage [1]. The findings on computerized tomography (CT) scan described by COVID-19 in order of frequency are ground glass opacities, consolidation, air bronchograms, linear opacities, crazy-paving pattern, interlobular septal thickening, traction bronchiectasis, lymphadenopathies and pleural effusion in late stages in severe forms [2,4,6]. The appearance of pulmonary cavitations is uncommon and their pathophysiology is not clarified [2,3,4,6]. Some retrospective analyses suggest a frequency of 1.7 to 11 % of patients, depending mainly on the severity of the respiratory infection [6,7].
Case Report
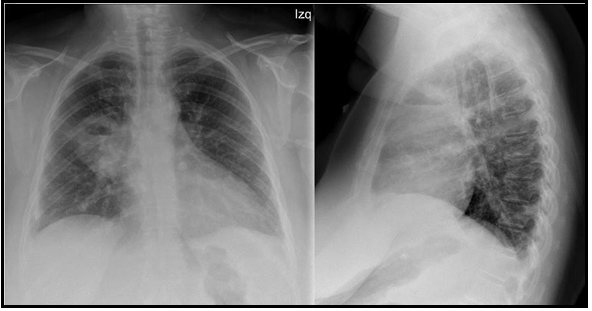
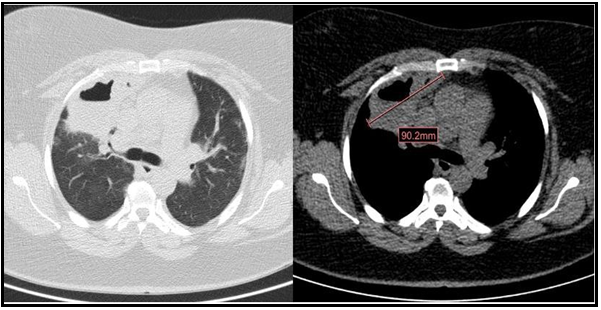
We present a 47-year-old active smoker with a history of asthma treated with formoterol/beclomethasone 400/12μcg daily, type 2 diabetes mellitus, and obesity. The patient had gone to the emergency department three times for asthma attacks treated with 60 mg of intravenous methylprednisolone, 30mg of oral methylprednisolone for 7 days, and 800 μcg of inhaled budesonide with the need for hospital admission due to acute respiratory failure. At the last admission, the nasopharyngeal exudate polymerase chain reaction (PCR) was performed, which was positive for respiratory infection by SARS-CoV-2. The chest x-ray showed parenchymal opacities of basal and peripheral predominance compatible with COVID-19 pneumonia. The patient required oxygen therapy in conventional nasal glasses at 2 liters per minute (l/min) and 30mg of oral methylprednisolone with good clinical evolution, so she was discharged at 6 days. She came back to the emergency department at 3 weeks for clinical worsening with dyspnea (grade 4 mMRC), fever of 37.8ºC, right pleuritic pain, cough with homophobic and greenish sputum of 4 days of evolution. On physical examination, she was tachypneic (respiratory rate of 24 breaths per minute) with oxygen saturation (SpO2) of 90 %, tachycardic (heart rate of 111 beats per minute), and hemodynamically stable with a blood pressure of 122/69 mmHg. Pulmonary examination revealed wet crackles in the right upper hemithorax. Laboratory studies showed an elevation of c-reactive protein (CRP 109.5 mg/L) and leukocytosis with neutrophilia (leukocytes 13.10 10^3/μ, neutrophils 9.75 10^3/μ). At this time, nasopharyngeal exudate PCRs were repeated for SARS- CoV-2, respiratory syncytial virus, and influenza A virus that was negative. The chest X-ray showed an acute-looking parenchymal opacity with a hydrothermal level in the right upper lobe (Figure 1). Because of the suspicion of cavitated pneumonia versus pulmonary abscess, empirical broad-spectrum antibiotic therapy with piperacillin-tazobactam was initiated and she was admitted to the pulmonology department. CT of the chest showed a cavitated-abscess pulmonary consolidation in the right upper lobe of 5.3x9mm and pulmonary peripheral alveolar opacities suggestive of an infectious-inflammatory process (Figure 2). Sputum culture tested positive for Staphylococcus aureus. Urinary antigen tests for Pneumococcus and Legionella were negative. Serologies for HIV and other viruses were negative as well. No bronchoscopy was performed. The patient presented a satisfactory clinical evolution. A reduction of the pulmonary infiltrate was observed in the control x-ray at 11 days. She received supplemental oxygen therapy up to a maximum flow of 2 l/min. She left the hospital 15 days after admission with oral antibiotic therapy and follow-up consultations. On the day of the discharge, her SpO2 breathing ambient air was 95%.
Figure 1. Posteroanterior and lateral radiography of the chest images. Acute parenchymal opacity with hydro aerial level in the right upper lobe.
Figure 2. Computerized tomography (CT) scan images of the chest images. Cavitated-abscess pulmonary consolidation in the right upper lobe of 5.3 x 9 mm and bipulmonary peripheral alveolar opacities suggestive of an infectious-inflammatory process.
Pulmonary cavitation is defined as the air-filled space formed within an area of consolidation, mass, or pulmonary nodule, resulting from the liquefaction of the necrotic portion of a lesion and the discharge of this necrotic material via the bronchial tree [2,4].
In chest CT, the characteristic alterations of SARS-CoV-2 infection are the ground-glass opacities, consolidative pulmonary infiltrates predominantly in the lower lobes with a peripheral distribution2. Pulmonary cavitations are uncommon in viral respiratory infections, even in severe forms of disease [2,4]. There is no specific treatment for severe COVID-192. The most used drugs are redelivered, tocilizumab and corticosteroids [2,4]. Corticosteroids are the only drugs that improve survival; however, they suppress the immune system favoring superinfection2,4. Our patient received intravenous, oral, and inhaled corticosteroid therapy in addition to being an immunocompromised patient.
The development of pulmonary cavitations in patients who have passed a recent SARS-CoV-2 infection is not clear [2,3,4,6]. A multifactorial origin consisting of bacterial or fungal superinfection/coinfection is proposed, favored by the immunosuppressive effects of corticosteroids, the activation of SARS-CoV-2 specific inflammatory cascades, the predisposition to develop the venous thromboembolic disease, as well as the morbidity of patients [2,4,6]. COVID-19 produces a prothrombotic state related to venous thromboembolic events that can lead to pulmonary infarctions, liquefaction, and predisposition to the formation of cavitation On the other hand, the coronavirus causes a serious alteration of the immune reaction of the host that injures the cells of the bronchial endothelium, causing necrosis and cellular apoptosis and diffuse alveolar damage that would also favor the appearance of cavitations [2,3,4,5,6]. The space filled with air can be the result of a pathological dilation of a physiological space or be associated with a process of consolidation and subsequent resorption [3,4]. This process probably occurred in our patient because the cavity was formed in the area of the lung where the consolidation was in previous stages. Because the patient did not require high-flow oxygen therapy or non-invasive mechanical ventilation, iatrogenesis by ventilation was not contemplated [3]. The origin of cavitations in those patients with a mild coronavirus infection may be due to the long-lasting mucous content in the bronchioles along with the consolidations and inflammatory process in the parenchyma, responsible for inflammatory damage and widening in the bronchiole leading to fibrosis, traction bronchiectasis and finally cavitations [6].
The treatment of choice, given the suspicion of superinfection, is broad-spectrum antibiotic therapy [2]. In cases of torpid evolution, the placement of an endothoracic drainage is indicated [2]. Surgical resection is uncommon due to the high mortality resulting from intra- and postoperative complications [2]. Morbidity and mortality after the appearance of cavitations are high (30 %) and they usually appear in the last stages of the disease [2]. They can cause serious complications such as hemoptysis and secondary pneumothorax, being independent of the size of the cavitation [2,4]. It can also lead to colonization by bacteria including nontuberculous mycobacteria, and fungi, and develop mycetomas [4].
The complications associated with COVID-19 disease are not yet known and their spectrum is growing [2]. We present this clinical case, due to the exceptionality of pulmonary cavitations as a late complication of COVID-19, which must be detected early to rule out added superinfections or complications that may worsen the patient's prognosis [2,3,4,6].
References
- Parra Gordo ML, Weiland GB, García MG, Choperena GA (2021) Aspectos radiológicos de la neumonía COVID-19: evolución y complicaciones torácicas. Radiol (Engl Ed). 63(1): 74–88.
- Lozano Gómez H, Herrero García S, Arche Banzo MJ, Villanueva Anadón B, Melé D, et al. (2022) Cavitaciones pulmonares, complicación tardía de la COVID-19. Anales del Sistema Sanitario de Navarra. 45(1): e0974.
- Muheim M, Weber FJ, Muggensturm P, Seiler E (2020) An unusual course of disease in two patients with COVID-19: pulmonary cavitation. BMJ Case Rep. 13(9): e237967.
- Zoumot Z, Bonilla M-F, Wahla AS, Shafiq I, Uzbeck M, et al. (2021) Pulmonary cavitation: an under-recognized late complication of severe COVID-19 lung disease. BMC Pulm Med. 21(1): 24.
- Arias F, Chiappe A, Rey de Castro J, Zagaceta J (2021) Infected cavitations, bullae and interstitial lung disease in a COVID-19 patient in Lima, Peru. Eur J Case Rep Intern Med. 8(12): 003004.
- Kurys-Denis E, Grzywa-Celińska A, Celiński R (2021) Lung cavitation as a consequence of coronavirus-19 pneumonia. Eur Rev Med Pharmacol Sci. 25(19): 5936–41.
- Kruse JM, Zickler D, Lüdemann WM, Piper SK, Gotthardt I, et al. (2021) Evidence for a thromboembolic pathogenesis of lung cavitations in severely ill COVID-19 patients. Sci Rep. 11(1): 16039.