Irene Hidalgo-Hernández, MD1, Vilma Pacheco-Barcia PhD, MD2, Sara Custodio-Cabello MD, PhD2, Magda Palka-Kotlowska MD2, Fátima Carrasco-Valero, MD1, José Ramón Sevilla-Resua MD1, Sheherezade Gallego-Nieto MD1, Lucas Miragaya- Calderón MD1, Luis Cabezón-Gutiérrez MD, PhD2,3*
1Internal Medicine, Hospital Universitario De Torrejón, Madrid, Spain
2Medical Oncology, Hospital Universitario De Torrejón, Madrid, Spain
3Universidad Francisco de Vitoria. Ctra. Pozuelo-Majadahonda KM 1.800. 28223 Pozuelo de Alarcón (Madrid, Spain).
*Corresponding Author: Luis Cabezón-Gutiérrez. MD. PhD, Medical Oncology, Hospital Universitario De Torrejón, Madrid, Spain, Universidad Francisco de Vitoria. Ctra. Pozuelo-Majadahonda KM 1.800. 28223 Pozuelo de Alarcón (Madrid, Spain). ORCID: https://orcid.org/0000-0002-3468-3626
Abstract
Introduction: Thymic carcinomas are aggressive tumors. Approximately one-third of patients are newly diagnosed with metastatic disease and require systemic therapy. There is no standard treatment after progression to first-line platinum-based chemotherapy, although chemotherapy-immunotherapy and/or antiangiogenic strategies are being investigated.
Case presentation: We present an exceptional case of a patient with metastatic thymic carcinoma with extended survival who received lenvatinib as a fourth-line palliative treatment. Lenvatinib was well tolerated, and partial response was observed.
Discussion: No randomized clinical trials or guidelines have provided definitive guidance for the management of thymic carcinomas. Many studies and practice guidelines recommend platinum-based chemotherapy as first-line chemotherapy; however, a standard second-line treatment has not been established. As several patients are candidates for multiple lines of treatment, it is essential to obtain a complete genetic sequence profile to identify specific driver mutations that could help in the future to select targeted therapies that improve the prognosis of patients. Lenvatinib and sunitinib have shown efficacy in pre-treated patients with unresectable advanced thymic carcinomas.
Conclusions: Lenvatinib and sunitinib are options for unresectable advanced thymic carcinoma after progression to first-line treatment with platinum combination therapy for unresectable advanced thymic carcinoma.
Keywords: Thymic carcinoma, lenvatinib, multi-targeted kinase inhibitors, and JAK2-V617F mutation.
Introduction
Thymic epithelial neoplasms encompass different entities, including thymomas, thymic carcinomas, and thymic neuroendocrine neoplasms (NET), which represent approximately 50%, 14%-22%, and 2%-5% of thymic epithelial neoplasms, respectively [1].
The mean age at diagnosis is 40-50 years, with similar incidence in men and women. The pathogenesis is unkonwn. No environmental or infectious risk factors have been associated with this tumor, although, in many cases, thymic carcinomas can be associated with myasthenia gravis and different paraneoplastic syndromes [2].
Thymomas and thymic carcinomas could present as incidental findings, local symptoms (chest pain, cough, shortness of breath, superior vena cava syndrome), or associated symptoms from a paraneoplastic syndrome.
Extrathoracic metastases in thymic carcinomas are seen in fewer than 7% of patients at presentation, most commonly to the liver, kidney, brain, extrathoracic lymph nodes, thyroid, and bone [3].
Multidisciplinary management (clinicians, surgeons, and pathologists) of thymic epithelial tumors is essential due to the lack of definitive treatment guidelines, especially in the metastatic disease setting.
Approximately one-third of patients with newly diagnosed thymic carcinoma present with metastatic or unresectable disease [4].
Five-year survival of patients with TNM stage III or IV thymic carcinoma ranges from 67% (for individuals who have undergone complete surgical resection) to 24% (patients with inoperable disease) [5].
Platinum-based combination chemotherapy is recommended for the treatment of unresectable thymic carcinoma. Most often used regimens include a combination of cisplatin, doxorubicin, and cyclophosphamide, cisplatin with etoposide, or carboplatin with paclitaxel [5,6], with no significant differences in terms of survival between different chemotherapy combinations [7].
In the second-line setting, a definitive treatment approach is lacking due to the limited evidence for systemic treatment after progression to platinum-based chemotherapy. Strategies to combine chemotherapy with immunotherapy and antiangiogenics are under evaluation.
Tyrosine kinase inhibitors (TKIs) that inhibit vascular endothelial growth factor receptor (VEGFR) signaling and tumor angiogenesis have demonstrated efficacy in advanced thymic carcinomas in progression to the first line to date.
Here, we report a patient with metastatic thymic carcinoma with an excellent evolution with multiple lines of systemic treatment, in which a JAK2 (Janus kinase-2) mutation was detected in liquid biopsy.
Case Presentation
A 73-year-old woman with multinodular goiter and depressive disorder with no other associated comorbidities was diagnosed in April 2014 with unresectable thymic carcinoma stage III by the Masaoka-Koga system (infiltration of large vessels) and mild superior vena cava syndrome.
The patient was attended to the internal medicine service with symptoms of facial swelling, dyspnea of moderate exertion, and a feeling of pressure on the face and chest during the previous month, without fever or skin lesions with a minor degree of superior vena cava syndrome (SVCS). The physical examination revealed facial and upper limb edema, redness of the cheeks, and a minor increase in jugular venous pressure.
The case was evaluated by the multidisciplinary oncological committee, considering the disease unresectable. Induction chemotherapy treatment was planned (doxorubicin 40 mg/m2 intravenous (IV) Day 1, cisplatin 40 mg/m2 IV Day 1, vincristine 0.6 mg/m2 IV Day 3, and cyclophosphamide 700 mg/m2 IV Day 4 every three weeks) with corticosteroids (dexamethasone 12 mg daily) for the SVCS.
After three cycles of chemotherapy, stabilization of the disease was obtained. The case was re-evaluated by the multidisciplinary oncology committee, and surgical resection was rejected.
External beam radiotherapy (5040 cGy in 28 fractions) to the mediastinal mass was administered. The CT performed two months after the completion of radiotherapy showed stable disease. Surgery was definitively dismissed, and radiological follow-up was decided.
Three months later, pleural and pericardial progression of the disease was observed. The first line of palliative chemotherapy with a carboplatin area under the curve (AUC) of 6 and paclitaxel 225 mg/m2 IV every 21 days was started. The patient received six cycles with acceptable tolerability, and stable disease was the best response assessed in computed tomography (CT) in September 2015.
Six months later, disease progression was observed, presenting new pleural and pericardial implants. The patient was asymptomatic, with an Eastern Cooperative Oncology Group (ECOG) scale performance status (PS) of 0 and a normal echocardiogram and blood test.
In January 2016, the second line of palliative treatment was started under exemption use, with 50 mg of sunitinib orally once a day in six-week cycles (four weeks of treatment followed by two weeks without treatment). After the first cycle, due to significant toxicity (G2 afebrile neutropenia, G1 thrombocytopenia, G1 hypertension, and G3 asthenia), it was necessary to lower the dose to 37.5 mg per day with improved to grade 1 toxicity, presenting the patient an ECOG PS of 0. The best overall response was a partial response, but after 25 cycles (in December 2017), tumor progression was observed, with hemorrhagic pleura effusion that required hospitalization and pericardiocentesis. The ECOG PS of the patient at the moment was 1.
In January 2018, a third line of palliative treatment under exemption use was started with oral capecitabine (650 mg/m2 twice daily on Days 1-14) and gemcitabine (1000 mg/m2 IV on Days 1 and 8) every three weeks. In April 2020, gemcitabine was stopped due to clinical stability, and the patient continued with capecitabine as a maintenance treatment. In July 2021, with progression-free survival (PFS) of more than 30 months, treatment with capecitabine was discontinued (therapeutic rest requested by the patient).
One year later, in July 2022, after a PFS of 42 months, disease progression was observed (hepatic and thoracic metastases).
Due to the chemotherapy-free interval of one year, we retreated the patient with capecitabine and gemcitabine with partial response in August 2022. From the fifth cycle, we continue with capecitabine in monotherapy for better tolerability, achieving stabilization of the disease until April 2023, with the appearance of cardiac tamponade requiring pericardiotomy with excellent recuperation.
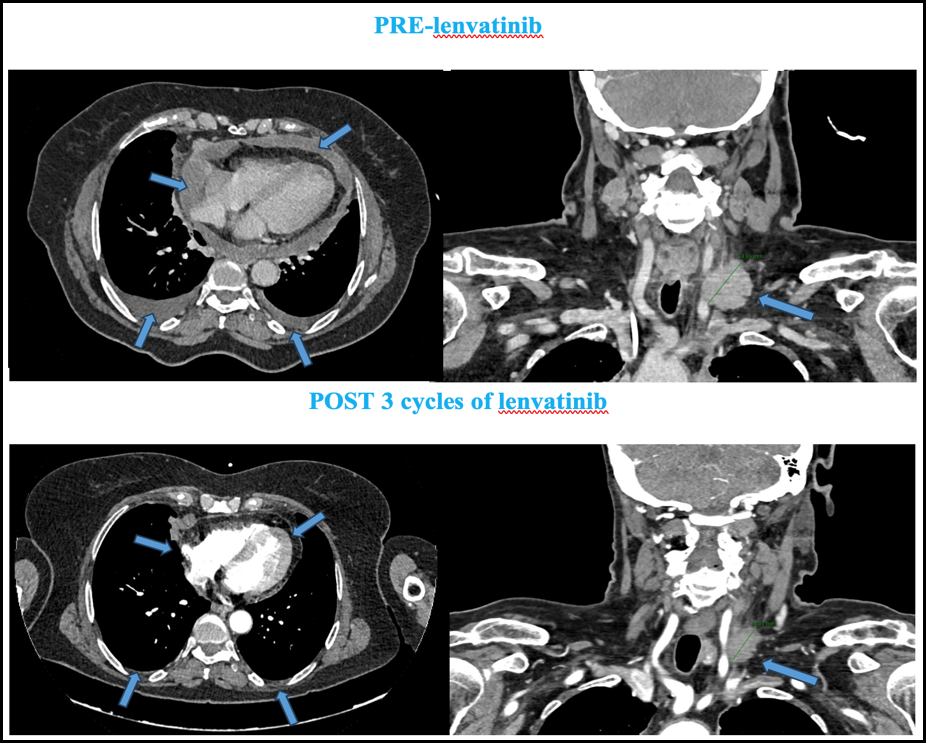
Figure 1: Abdominal-thoracic-cervical CT (computed tomography) scan after 3 months of lenvanitinib treatment. Partial response of the supraclavicular left adenopathy and complete response of pleural and pericardial effusion were obtained (blue arrows).
Due to the excellent clinical evolution, it was decided to perform a liquid biopsy. The FoundationOne Liquid CDx Ò assay is performed exclusively as a laboratory service using circulating cell-free DNA (cfDNA) isolated from plasma derived from anti-coagulated peripheral whole blood from patients with solid malignant neoplasms. The assay employs a single DNA extraction method to obtain cfDNA from plasma from whole blood. Extracted cfDNA undergoes whole-genome shotgun library construction and hybridization-based capture of 324 cancer-related genes, including coding exons and select introns of 309 genes, as well as only select intronic regions or non-coding regions of 15 genes.
A mutation in JAK2-V617F, 4 mutations per megabase, and microsatellite stable status (MSS) was obtained.
In May 2023, the fourth line of palliative treatment was started under exemption use with lenvatinib.
"Currently, the patient has ECOG PS 0, with excellent tolerability to three cycles of lenvatinib (only hypertension grade 1), and stabilization of lung mass with partial response of the supraclavicular left adenopathy and complete response of pleural and pericardial effusion was obtained." [Figure 1]
Figure 2: Summary of the treatment timeline and the clinical case course.
Abbreviations: PD: progression disease; PFS: progression-free survival.
Discussion
No randomized clinical trials or guidelines provide definitive guidance for the management of advanced thymic carcinomas. Some studies and practice guidelines recommend platinum-based chemotherapy as a first-line chemotherapy, but standard second-line treatment has not yet been established [8,9].
Many patients are candidates to receive multiple lines of treatment. There are Phase II trials that have demonstrated the efficacy of cytotoxic agents, immune checkpoint inhibitors, and molecular targeted drugs in unresectable advanced thymic carcinomas with different outcomes [10-12].
In this context, the drugs with the most evidence and efficacy in pre-treated patients are sunitinib and lenvatinib [13,14].
Lenvatinib is a novel orally administered multi-targeted kinase inhibitor for VEGFR, FGFR, c-Kit, and other kinases. It is involved in pathogenic angiogenesis, tumor growth, and cancer progression. It has been approved for the treatment of thyroid cancer, endometrial cancer, and hepatocellular cancer in several countries [15].
REMORA trial is a Japanese phase 2 trial in patients with pathologically confirmed unresectable advanced or metastatic thymic carcinoma. Patients received 24 mg of lenvatinib orally once daily in 4-week cycles until disease progression or occurrence of unacceptable adverse events. The primary endpoint was the objective response rate (ORR). 42 patients were enrolled. The median follow-up was 15·5 months. ORR was 38% (90% CI 25·6–52·0, p<0·0001). The disease control rate was 95%. The median progression-free survival (PFS) was 9.3 months (95% CI 7.7–13.9), and the median overall survival (OS) was not reached (NR; 95% CI 16.1–NR; figure 4). The probability of 12-month PFS was 41% (95% CI 25.8–54.7) and the probability of 12-month OS was 83% (68.2–91.7). The most frequent grade 3 treatment-related adverse events were hypertension (27 [64%]) and palmar-plantar erythrodysesthesia syndrome (three [7%]). No patient died from adverse events [16].
The combination of lenvatinib and pembrolizumab has also been investigated for several cancers, and this combination could be considered a potential candidate for treatment development for advanced or metastatic thymic carcinoma [17-19].
In our patient, the treatment with lenvatinib was started in May 2023. At the last visit, the patient maintained an ECOG PS of 0 and lived an everyday life. With only three cycles, stabilization of lung mass with partial response of supraclavicular left adenopathy and complete response of pleural and pericardial effusion was obtained.
It is also essential to know the different molecular pathways and their function in oncogenesis because this can help to discover new treatments. In our patient's tumor, a mutation in JAK2-V617F, 4 mutations per megabase, and microsatellite stable status (MSS) was obtained. As reflected in the Singh et al. [20] reviews, "the era of precision medicine is rapidly approaching, and the integration of personalized medicine, toxicology, toxicogenomics, and artificial intelligence can play a vital role in achieving its goals. These fields can help to optimize treatment outcomes and reduce the risk of adverse effects by taking into account an individual's unique characteristics such as genetics, lifestyle, and environment".
While JAK2 inhibitors have shown clinical benefit in hematological malignancies, clinical utility in solid tumors has not been demonstrated. JAK2 mutation has been detected in 1% of the thymus carcinoma samples analyzed in COSMIC [21]. Published data investigating the prognostic implications of JAK2 in thymus cancer are limited (PubMed, Sep 2023).
JAK2 encodes Janus kinase 2, a tyrosine kinase that regulates signals triggered by cytokines and growth factors [22]. JAK2 is often mutated in hematopoietic and lymphoid cancers. The JAK2 alteration observed here has been characterized as activating and is predicted to be oncogenic [22-34]. Limited clinical data suggest that JAK2 V617F mutations detected in solid tumors may originate from infiltrating hematopoietic cells and not the cancer itself [34].
Ruxolitinib is a selective inhibitor of JAK1 and 2 enzymes with potent immunosuppressive and anti-inflammatory properties on innate and adaptive immune effectors and reduces the secretion of several pro-inflammatory mediators, including IL-6 and TNF-alpha. It has demonstrated activity in hematological tumors (myelofibrosis, polycythemia vera), graft-versus-host disease, and solid tumors such as pancreatic cancer [35].
In a phase II study, Ruxolitinib in Pancreatic Cancer Patients (RECAP), the combination of ruxolitinib with capecitabine suggested an association with improved survival compared with placebo and capecitabine [36].
When our patient's tumor progresses to lenvatinib, the use of ruxolitinib under exemption will be considered. The sequence of target treatments could be key to obtaining patients with prolonged survival, as our clinical case demonstrates.
Conclusion
Lenvatinib is a subsequent line option after progression to first-line treatment with platinum combination therapy in unresectable advanced thymic carcinomas. Despite new molecular mechanisms involved in thymic carcinogenesis are known, it is necessary to continue investigating to develop better therapeutic strategies.
This case highlights the importance of obtaining a complete genetic sequence profile to identify specific driver mutations that could help in the future to select targeted treatments that improve the prognosis of our patients.
Conflict of Interest
Luis Cabezón Gutiérrez declares the following conflicts of interest: Advisory role; Astra Zeneca, Roche, Eisai, and Bristol Myers Squibb. Speakers' bureau; Roche, Astra Zeneca, Brystol Myers Squibb, Merck Serono, Ipsen Pharma, Grunenthal, Kyowa Kirin, Pfizer, Roche, and Eisai.
Vilma Pacheco-Barcia declares the following conflicts of interest: Advisory role: Advanced accelerator applications, a Novartis company; Speakers' bureau: Merck, Eli Lilly, Eisai, Pierre Fabre. Congress attendance: Roche, Eli Lilly, Bristol-Myers Squibb, Merck, Amgen, Merck Sharp and Dhome, Nutricia; Grant support: FSEOM and Merck, Pfizer, Nutricia, LEO Pharma. Other: Bayer, Roche, Amgen, Esteve.
Sara Custodio-Cabello, Magda Palka-Kotlowska, Irene Hidalgo-Hernández, Fátima Carrasco-Valero, José Ramón Sevilla-Resua, Sheherezade Gallego-Nieto and Lucas Miragaya-Calderón, declare no conflict of interests.
References
- Willner J, Zhou F, Moreira AL (2022) Diagnostic challenges in the cytology of thymic epithelial neoplasms. Cancers. 14(8): 2013.
- Safieddine N, Liu G, Cuningham K, Ming T, Hwang D, et al. (2014) Prognostic factors for cure, recurrence, and long-term survival after surgical resection of thymoma. J Thorac Oncol. 9(7): 1018-1022.
- Lewis JE, Wick MR, Scheithauer BW, Bernatz PE, Taylor WF (1987) Thymoma. A clinicopathologic review. Cancer. 60(11): 2727-43.
- Girard N, Ruffini E, Marx A, Faivre-Finn C, Peters S (2015) Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and followup. Ann Oncol. 26(suppl 5): v40–v55.
- Kondo K, Monden Y (2003) Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg. 76(3): 878-84; discussion 884-5.
- Berghmans T, Durieux V, Holbrechts S, Jungels C, Lafitte JJ, et al. (2018) Systemic treatments for thymoma and thymic carcinoma: a systematic review. Lung Cancer. 126: 25–31.
- Ko R, Shukuya T, Okuma Y, Tateishi K, Imai H, et al. (2018) Prognostic factors and efficacy of first-line chemotherapy in patients with advanced thymic carcinoma: A retrospective analysis of 286 patients from NEJ023 study. Oncologist. 23(10): 1210–1217.
- Ettinger DS, Riely GJ, Akerley W, Borghaei H, Chang AC, et al. (2013) Thymomas and thymic carcinomas: Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 11(5): 562– 76.
- Girard N, Ruffini E, Marx A, Faivre-Finn C, Peters S (2015) Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 26(Suppl 5): v40–55.
- Lemma GL, Lee JW, Aisner SC, Langer CJ, Tester WJ, et al. (2011) Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol. 29(15): 2060–65.
- Giaccone G, Kim C, Thompson J, McGuire C, Kallakury B, et al. (2018) Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. 19(3): 347–355.
- Katsuya Y, Horinouchi H, Seto T, Umemura S, Hosomi Y, et al. (2019) Single-arm, multicentre, phase II trial of nivolumab for unresectable or thymic carcinoma: PRIMER study. Eur J Cancer. 113: 78–86.
- Thomas A, Rajan A, Berman A, Tomita Y, Brzezniak C, et al. (2015) Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol. 16(2): 177–86.
- Kim SH, Kim YJ, Ock C, Kim M, Keam B, et al. (2018) OA11.05 phase II study of sunitinib in patients with thymic carcinoma previously treated with platinum-based chemotherapy (KOSMIC Trial). J Thorac Oncol. 13(10): S346–S347.
- Zhao Y, Zhang YN, Wang KT, Chen L (2020) Lenvatinib for hepatocellular carcinoma: From preclinical mechanisms to anti- cancer therapy. Biochim Biophys Acta Rev Cancer. 1874(1): 188391.
- Sato J, Satouchi M, Itoh S, Okuma Y, Niho S, et al. (2020) Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol. 21(6): 843–850.
- Ott PA, Hodi FS, Buchbinder EI. (2015) Inhibition of immune checkpoints and vascular endothelial growth factor as combination therapy for metastatic melanoma: an overview of rationale, preclinical evidence, and initial clinical data. Front Oncol. 5: 202.
- Makker V, Rasco D, Vogelzang NJ, Brose MS, Cohn AL, et al. (2019) Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open- label, single-arm, phase 2 trial. Lancet Oncol. 20(5): 711–718.
- Lee CH, Makker V, Rasco DW, Taylor MH, Stepan DE, et al. (2018) Lenvatinib + pembrolizumab in patients with renal cell carcinoma: updated results. J Clin Oncol. 36(15): 4560.
- Singh AV, Chandrasekar V, Paudel N, Laux P, Luch A, et al. (2023) Integrative toxicogenomics: Advancing precision medicine and toxicology through artificial intelligence and OMICs technology. Biomedicine & Pharmacotherapy. 163: 114784.
- Tate JG, Bamford S, Jubb HC, Sondka Z, Beare DM, et al. (2019) COSMIC: the Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 47(D1): D941-D947.
- Jatiani SS, Baker SJ, Silverman LR, Reddy EP (2010) Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 1(10): 979-93.
- Walz C, Crowley BJ, Hudon HE, Gramlich JL, Neuberg DS, et al. (2006) Activated Jak2 with the V617F point mutation promotes G1/S phase transition. J Biol Chem. 281(26): 18177-83.
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, et al. (2005) A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 352(17): 1779-90.
- Etheridge SL, Cosgrove ME, Sangkhae V, Corbo LM, Roh ME, et al. (2014) A novel activating, germline JAK2 mutation, JAK2R564Q, causes familial essential thrombocytosis. Blood. 123(7): 1059-68.
- Marty C, Saint-Martin C, Pecquet C, Grosjean S, Saliba J, et al. (2014) Germ-line JAK2 mutations in the kinase domain are responsible for hereditary thrombocytosis and are resistant to JAK2 and HSP90 inhibitors. Blood. 123(9): 1372-83.
- Ma W, Kantarjian H, Zhang X, Yeh CH, Zhang ZJ, et al. (2009) Mutation profile of JAK2 transcripts in patients with chronic myeloproliferative neoplasias. J Mol Diagn. 11(1): 49-53.
- Mullighan CG, Zhang J, Harvey RC, Collins-Underwood JR, Schulman BA, et al. (2009) JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 106(23): 9414-8.
- Yoda A, Yoda Y, Chiaretti S, Bar-Natan M, Mani K, et al. (2010) Functional screening identifies CRLF2 in precursor B-cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 107(1): 252- 7.
- Amrolia PJ, Muccioli-Casadei G, Huls H, Adams S, Durett A, et al. (2006) Adoptive immunotherapy with allodepleted donor T- cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 108(6): 1797-808.
- Funakoshi-Tago M, Tago K, Sumi K, Abe M, Aizu-Yokota E, et al. (2009) The acute lymphoblastic leukemia-associated JAK2 L611S mutant induces tumorigenesis in color piel mice. J Biol Chem. 284(19): 12680-90.
- Grisouard J, Li S, Kubovcakova L, Rao TN, Meyer SC, et al. (2016) JAK2 exon 12 mutant mice display isolated erythrocytosis and changes in iron metabolism favoring increased erythropoiesis. Blood. 128(6): 839-51.
- Scott LM, Tong W, Levine RL, Scott MA, Beer PA, et al. (2007) JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 356(5): 459-68.
- Nguyen QN, Chun SG, Chow E, Komaki R, Liao Z, et al. (2019) Single-Fraction Stereotactic vs Conventional Multifraction Radiotherapy for Pain Relief in Patients With Predominantly Nonspine Bone Metastases: A Randomized Phase 2 Trial. JAMA Oncol. 5(6): 872-878.
- Elli EM, Baratè C, Mendicino F, Palandri F, Palumbo GA (2019) Mechanisms Underlying the Anti-inflammatory and Immunosuppressive Activity of Ruxolitinib. Front Oncol. 9: 1186.
- Hurwitz HI, Uppal N, Wagner SA, Bendell JC, Beck JT, et al. (2015) Randomized, Double-Blind, Phase II Study of Ruxolitinib or Placebo in Combination with Capecitabine in Patients with Metastatic Pancreatic Cancer for Whom Therapy with Gemcitabine Has Failed. J Clin Oncol. 33(34): 4039-47.