Luke Bugeja1*, Scicluna C1, Pisani D2, Vella M1, Galea A1, Sciberras N1, Chetcuti Zammit S1
1Department of Gastroenterology and Immunology, Mater Dei Hospital Malta
2Department of Histopathology, Mater Dei Hospital Malta
*Corresponding Author: Luke Bugeja, Department of Gastroenterology and Immunology, Mater Dei Hospital Malta
Abstract
Background: Gastric MALT lymphoma is a rare extra-nodal B-cell lymphoma strongly linked to Helicobacter pylori infection. While H. pylori eradication induces remission in most early-stage cases, the relationship between infection status and disease extent or prognosis remains unclear.
Methods: We conducted a retrospective analysis of all patients diagnosed with primary gastric MALT lymphoma in Malta between 2008 and 2022. Clinical data including H. pylori status, presence of lymphadenopathy, extra-nodal involvement, sex, management, and outcomes were reviewed. Statistical analysis was performed using non-parametric tests, with significance set at p < 0.05.
Results: Eighteen patients (55.5% female; median age 69.5 ± 11.4 years) were included in this study. H. pylori was detected in 50% via CLO testing and histology. No significant association was found between H. pylori status and lymphadenopathy (p = 0.563), extra-nodal involvement (p = 0.352), or sex and prognosis (p = 0.108). Follow-up endoscopies and biopsies varied, reflecting personalized surveillance strategies.
Conclusion: In this cohort, H. pylori status and sex did not predict disease dissemination or prognosis. Findings support rigorous surveillance regardless of infection status and highlight the need for larger, multicentre studies to further clarify prognostic factors and microbial contributions to gastric MALT lymphoma.
Keywords: MALT lymphoma; H Pylori; Oncology
Introduction
Gastric Mucosa-associated lymphoid tissue (MALT) lymphoma is an extranodal B-cell lymphoma strongly associated with Helicobacter pylori (H. pylori) infection. Approximately 90% of patients affected with MALT lymphoma are found to be H. Pylori positive. [1] Although initially they were reported to be a subtype of gastric lymphoma, MALT lymphomas have subsequently been shown to arise in all organs of the human body, including unusual sites such as the dura. Although the World Health Organization classification published in 2008 still defines the stomach as the most common organ of origin (accounting for 50% of MALT lymphomas), recent data have suggested a decline in the percentage of gastric MALT lymphomas. [2]
The recurrence after successful therapy of extra-gastric MALT lymphomas appears to be significantly higher compared with their gastric counterparts [3, 4]. A difference between the clinical presentation of gastric versus non-gastric MALT lymphoma is shown with a high rate of multiorgan involvement (32%) and dissemination (26% had stage IV disease) at diagnosis of extra-nodal non-gastric MALT lymphoma. Primary gastric lymphoma (PGL) is not a common pathology, and histologically, can vary from indolent marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue (MALT) to aggressive diffuse large B-cell lymphoma (DLBCL). During the years, a clear association between Helicobacter pylori (H. pylori) and gastric MALT lymphoma has been described. Long-term studies confirm that eradication therapy can regress gastric lymphomas. [5]
In this study, all patients diagnosed with primary gastric MALT lymphoma in Malta between 2008 and 2022 were assessed. Presence of Helicobacter Pylori, management, lymphadenopathy, extra-nodal involvement and prognosis was reviewed.
Our aim was to understand the association of Helicobacter Pylori and primary gastric MALT lymphoma, and how it affects management and clinical outcomes. Our secondary aim was to assess whether there would be any statistical significance in the sex of patients and their prognosis.
Materials And Methods
Study Design And Patients
This was a retrospective study investigating the relationship between gastric MALT lymphoma and H. Pylori in Malta between the years 2008 and 2022.
Inclusion And Exclusion Criteria
All patients with a diagnosis of gastric MALT lymphoma between 2008 and 2022 in Malta were included in this study. Demographic data and information regarding investigations was collected.
Statistical Analysis
Statistical analysis was carried out using SPSS version 28 (IBM Corp. Released 2015. IBM SPSS Statistics for Mac, Version 28.0. Armonk, NY: IBM Corp.). Frequencies, medians, standard deviations and interquartile range (IQR) were calculated to characterize the cohort studied. Non-parametric statistical tests were used namely, the Mann- Whitney U test to compare two independent, continuous variables. Fisher's Exact Test was utilized to assess the relationship between independent, discrete variables and categorical variables. Results were statistically significant if the p-value was less than 0.05.
Ethical Considerations
Data protection clearance was obtained from the local research and development team within Mater Dei Hospital.
Results
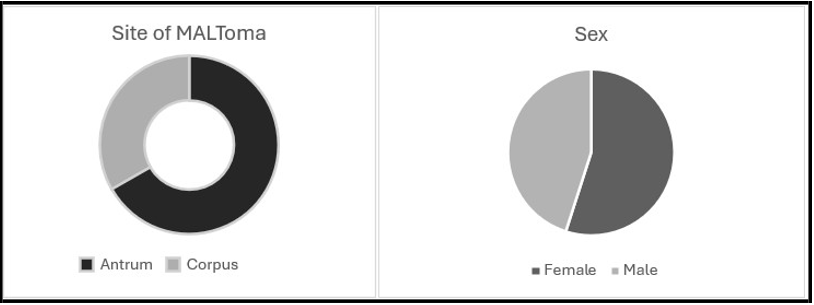
Data collection over 14 years was carried out, and a total of eighteen patients were included in the study (55.5% female), with a median age of 69.5 years (±11.38). Helicobacter pylori was detected in 9 patients (50%) using both the Campylobacter-like organism (CLO) test and histopathological analysis. All patients were treated with H Pylori eradication, regardless of their H Pylori status. 38.8% (n= 7) of patients were only treated with H Pylori eradication therapy, whilst 61.2% (n= 11) required chemotherapy, radiotherapy or both.
No statistically significant association was found between H. pylori status and the presence of lymphadenopathy (p = 0.563) or extra- nodal involvement (p = 0.352).
Similarly, no significant difference was observed between patient sex and prognosis (p = 0.108).
66.6% of MALT lymphomas were found in the antrum (n=12), whilst 33.4% were found in the pylorus (n=6).
Patients underwent an average of 3.2 follow-up endoscopies (range: 0–11). Across these procedures, an average of 6.5 biopsies were taken per endoscopy, with the number ranging from 1 to 17 biopsies per session. Only 0.05% (n=1) of patients had appropriate endoscopic mapping done according to ESMO guidance.
Discussion
Gastric mucosa-associated lymphoid tissue (MALT) lymphoma is a clinically indolent B-cell neoplasm that often remains localized for prolonged periods, in contrast to nodal lymphomas, which more frequently exhibit systemic spread. The disease arises from post- germinal centre memory B cells that can differentiate into marginal zone and plasma cells under chronic antigenic stimulation. Persistent inflammation, driven by microbial, viral, or autoimmune triggers promote local accumulation of antigen-dependent B‑ and T‑cell clones. Initially, neoplastic proliferation remains confined to the inflamed tissue, however, acquisition of secondary genetic alterations confers antigen-independence and facilitates dissemination.
Four recurrent chromosomal translocations have been identified in MALT lymphoma pathogenesis: t (11;18) (q21; q21), t (14;18) (q32; q21), t (1;14) (p22; q32), t (3;14) (p13;q32) [6].
Helicobacter pylori infection is the most well‑characterized etiologic factor in gastric MALT lymphoma. The bacterium induces the accumulation of CD4+ T cells and mature B cells in the gastric lamina propria.
H. pylori antigens subsequently drive T-cell activation, B-cell proliferation, and lymphoid follicle formation, which may evolve into monoclonal lymphoma under persistent stimulation. Although other bacterial species (e.g., Chlamydia psittaci, Campylobacter jejuni, Borrelia afzelii, Achromobacter xylosoxidans) have been implicated in extra-gastric MALT lymphomas, their role in gastric disease remains less defined.
In healthy individuals, gastric lymphoid tissue is absent; its emergence in H. pylori infection underscores the bacterium’s unique capacity to elicit mucosal immune responses. Notably, H. pylori stimulates the proliferation of low‑grade gastric B‑cell MALT lymphomas, whereas such effects are not observed in non-gastric sites nor with other bacterial species [7, 8, 9].
Despite the aetiologic association, approximately 10% of gastric MALT lymphomas are H. pylori negative. Remarkably, eradication therapy yields remission in a subset of these patients, possibly by eliminating occult non–H. pylori pathogens. Overall, H. pylori eradication achieves complete remission in ~75% of positive cases, with the remainder requiring radiotherapy, chemotherapy, or immunotherapy (e.g., anti-CD20 monoclonal antibodies). Patients with minimal residual disease after eradication often follow an indolent course, supporting a watch-and-wait approach with periodic endoscopy and biopsy [10-16].
In our single-centre cohort of eighteen patients (55.5% female; median age 69.5 ± 11.4 years), 50% tested positive for H. pylori by both CLO test and histology. This prevalence aligns with reported rates of 50–60%. We observed no significant correlation between H. pylori status and lymphadenopathy (p = 0.563) or extra‑nodal involvement (p = 0.352), suggesting that once established, disease extent may be influenced more by host and genetic factors than ongoing infection. Sex was not a predictor of prognosis (p = 0.108), consistent with larger retrospective analyses showing minimal sex- based outcome differences; however, our limited sample size may preclude detection of subtle effects.
Follow-up was intensive, with a mean of 3.2 endoscopies per patient (range 0–11) and 6.5 biopsies per procedure (range 1–17), reflecting individualized surveillance strategies for residual or recurrent disease. These data reinforce guidelines advocating systematic endoscopic assessment and targeted biopsies irrespective of initial H. pylori status.
Limitations
Limitations of this study include its retrospective design, single institution setting, and small sample size, which may limit generalizability and statistical power. Future prospective, multicentre studies should incorporate standardized staging, molecular risk markers (e.g., t (11;18)), and detailed assessment of microbial virulence factors to refine patient stratification and optimize therapeutic and surveillance algorithms.
Summary Box
Helicobacter pylori (H. pylori) infection has long been implicated in the development of mucosa-associated lymphoid tissue (MALT) lymphoma. This retrospective study examines a nationwide cohort of patients diagnosed with MALT lymphoma over a 14-year period. Despite the well-established association between H. pylori and gastric MALT lymphoma, only 50% of patients in our cohort tested positive for the bacterium, although all received H. pylori eradication therapy as part of their management. Importantly, neither H. pylori status nor sex were found to be predictive of disease dissemination or prognosis. These findings emphasize the importance of regular, personalized endoscopic surveillance in the management of MALT lymphoma, particularly for detecting residual or recurrent disease, regardless of H. pylori status.
Conclusion
In our retrospective analysis, neither Helicobacter pylori status nor patient sex was associated with disease dissemination or prognosis in MALT lymphoma. Endoscopic surveillance post treatment of H Pylori according to European guidelines remains critical for detecting residual or recurrent disease and informing subsequent management decision.
References
- Asano N, Iijima K, Koike T, Imatani A, Shimosegawa T (2015) Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphomas: A review. World J Gastroenterol. 21(26): 8014-20.
- Raderer M, Wöhrer S, Kiesewetter B, Dolak W, Lagler H, et al. (2015) Antibiotic treatment as sole management of Helicobacter pylori-negative gastric MALT lymphoma: a single center experience with prolonged follow-up. Ann Hematol. 94(6): 969- 73.
- Wöhrer S, Kiesewetter B, Fischbach J, Müllauer L, Troch M, et al. (2014) Retrospective comparison of the effectiveness of various treatment modalities of extragastric MALT lymphoma: a single-center analysis. Ann Hematol. 93(8): 1287-95.
- Raderer M, Wöhrer S, Streubel B, Troch M, Turetschek K, et al. (2006) Assessment of disease dissemination in gastric compared with extragastric mucosa-associated lymphoid tissue lymphoma using extensive staging: a single-center experience. J Clin Oncol. 24(19): 3136-41.
- Wang YG, Zhao LY, Liu CQ, Pan SC, Chen XL, et al. (2016) Clinical characteristics and prognostic factors of primary gastric lymphoma. Medicine (Baltimore). 95(31): e4250.
- Mucosa-associated lymphoid tissue (MALT) lymphoma is a heterogeneous form of a B-cell non-Hodgkin's lymphoma with extranodal location. The gastrointestinal tract is the most common site of disease, but involvement of multiple other organ systems has been documented. Four translocations, t (11;18) (q21; q21), t (1;14) (p22; q32), t (14;18) (q32;q21) and t(3;14)(p13;q32), are specifically associated with MALT lymphoma. Remarkably, the genes targeted by at least three of these translocations are involved in one and the same pathway, leading to the activation of nuclear factor-kappaB (NF-kappaB). This review presents MALT lymphoma as a model of how sustained inflammation increases the risk of genotoxic insults and how these genetic events initiate oncogenesis.
- Kuo SH, Cheng AL (2013) Helicobacter pylori and mucosa- associated lymphoid tissue: what's new. Hematology Am Soc Hematol Educ Program. 2013: 109-17.
- Adam P, Czapiewski P, Colak S, Kosmidis P, Tousseyn T, et al. (2014) Prevalence of Achromobacter xylosoxidans in pulmonary mucosa-associated lymphoid tissue lymphoma in different regions of Europe. Br J Haematol. 164(6): 804-10.
- Wyatt JI, Rathbone BJ (1988) Immune response of the gastric mucosa to Campylobacter pylori. Scand J Gastroenterol. 142: 44- 9.
- Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG (1991) Helicobacter pylori-associated gastritis and primary B-cell gas-tric lymphoma. Lancet. 338(8776): 1175-6.
- Wotherspoon AC, Finn T, Isaacson PG (1994) Numerical abnormalities of chromosome 3 and 7 in lymphomas of mucosa associated lymphoid tissue and the splenic marginal zone. LabInvest. 70: 124A.
- Nakamura S, Matsumoto T, Ye H, Nakamura S, Suekane H, et al. (2006) Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: a clinicopathologic and molecular study with reference to antibiotic treatment. Cancer. 107(12): 2770-8.
- Morgner A, Lehn N, Andersen LP, Thiede C, Bennedsen M, et al. (2000) Helicobacter heilmannii-associated primary gastric low- grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 118(5): 821-8.
- Nakamura S, Sugiyama T, Matsumoto T, Iijima K, Ono S, et al. (2012) Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut. 61(4): 507-13.
- Zucca E, Conconi A, Laszlo D, López-Guillermo A, Bouabdallah R, et al. (2013) Addition of rituximab to chlorambucil produces superior event-free survival in the treatment of patients with extranodal marginal-zone B-cell lymphoma: 5-year analysis of the IELSG-19 Randomized Study. J Clin Oncol. 31(5): 565-72.
- Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, Wündisch T, Neubauer A, et al. (2007) EGILS (European Gastro- Intestinal Lymphoma Study) Group. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut. 56(12): 1685-7.