Ahmed Aly, MD1, Tarek Aly, MD, PhD.2*
1Tanta University Hospitals
2Orthopedic department, Tanta university, Faculty of medicine, 48th Sarwat street, Tanta 31111, Egypt.
*Corresponding Author: Tarek Aly, MD, PhD, Orthopedic department, Tanta university, Faculty of medicine, 48th Sarwat street, Tanta 31111, Egypt.
Abstract
Lumbar spinal stenosis is a common bone disease in the elderly. The thickness of ligamentum flavum plays a significant role in the pathogenesis of lumbar spinal stenosis. Lumbar spine stenosis secondary to Ligamentum flavum hypertrophy is expected in older people, most of whom are in the L4/5 level. Ligamentum flavum hypertrophy was associated with quantitative and qualitative lipid changes and demonstrated total lipid accumulation in hypertrophied ligamentum flavum patients. Many researchers suggest a relation between the severity of COVID-19 and the extent of phospholipid changes.
This study presents the case of a 27-year-old female patient with Lumbar spinal canal stenosis with hypertrophy of ligamentum flavum following affection with COVID-19 coronavirus.
Keywords: Ligamentum flavum, Lumbar spinal stenosis, Covid19, Coronavirus.
Introduction
Lumbar spinal stenosis is one of the most common spinal degenerative diseases, and it has a high incidence among elderly individuals. Lumbar spinal stenosis is usually caused by hypertrophied ligamentum flavum [1-4]. Thickening of ligamentum flavum can cause spinal stenosis and compress the nerve roots or cauda equina, leading to back pain and intermittent claudication [5]. Various factors, including age, activity level, genetic composition, and mechanical stress, accelerate the development of hypertrophied ligamentum flavum [6,7]. Most researchers agree that an abnormal stress level can accelerate the degradation and hypertrophy of ligamentum flavum [8-10].
The average human ligamentum flavum is an elastic structure of 80% and 20% collagen fibers [11]. Increased collagen fibers, broken elastic fibers, and increased cell numbers in human hypertrophied ligamentum flavum are associated with the increased expression of fibrosis-related factors [12,13].
The pathogenesis of thickening of the ligamentum flavum is not clear. There is still controversy about whether the thickening is due to tissue hypertrophy or deformation. Some studies state that the narrowing of the canal results from the ligament's hypertrophy, while others argue that deformities of the ligamentum flavum inside the spinal canal compress the nerve tissues [14,15]. Additionally, the terms "thickening" and "hypertrophy" are used interchangeably in the literature, although they are not necessarily the same thing [16]. Sakamaki described the thickening of the ligamentum flavum as being caused by hypertrophied or deformed tissue, and they questioned whether it was related to disc degeneration in the MRI exams [15].
The relationship between the thickness of the ligamentum flavum, the degeneration of the intervertebral disc, and disc height at L3-L4, L4- L5, and L5-S1 was examined, and the investigators confirmed that the thickness of the ligamentum flavum at levels L3-L4, L4-L5, and L5- S1 was not significantly greater in patients with grade IV and V degeneration than in those with grade I to III degeneration. These results suggest that the thickening of the ligamentum flavum is not due to the deformation of this ligament inside the vertebral canal because of disc degeneration [17].
We presented a case of hypertrophy of ligamentum flavum with manifestations of lumbar spinal stenosis following Coronavirus infection.
Case Presentation
A 27-year-old female presented with complaints of radiating pain along the back of both thighs, more on the right side than the left, for 2 months. The patient described the pain as a sharp shooting sensation in the back of the thighs, more on the right side. The pain is associated with numbness and tingling in both the lower extremities. A worsening numbness in both legs (right more than left) was also described. The patient had tried various conservative measures for lower back pain. Decreased walking distance with intermittent claudication was also reported. The patient denied any recent onset of bowel or bladder dysfunction.
The patient had a recent onset of fever, with flu-like symptoms 3 months before the back and leg symptoms. The diagnosis of Coronavirus was confirmed.
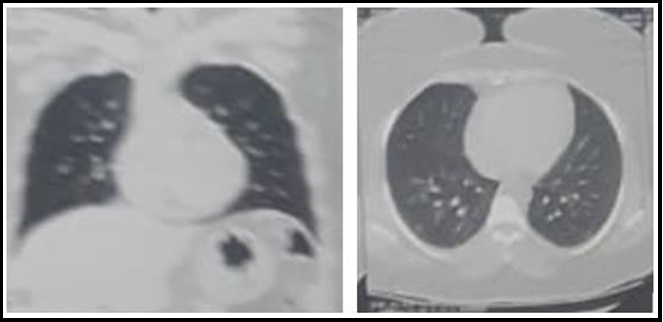
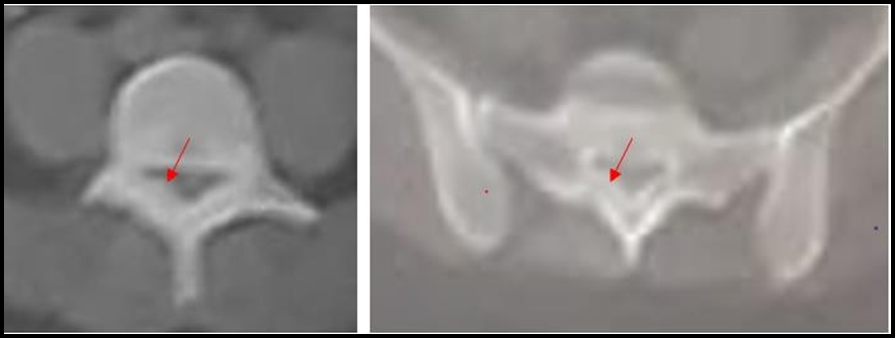
CT chest was done and confirmed CORADS 4th degree affection of the lungs with ground glass appearance. CT of the lumbar spine showed marked hypertrophy of ligamentum flavum at L5-S1 (4.7 mm on the right side) level with narrowing of the spinal canal to 8.5 mm in AP diameter at the same level.
Figure 1: Ground glass appearance of both lungs on CT scan.
Figure 2: CT scan of L5-S1 level showing marked hypertrophy of ligamentum flavum with narrowing of spinal canal (Red arrow).
Discussion
Lumbar spinal canal stenosis is a common cause of low back and lower extremity pain, particularly in elderly patients (18). Hypertrophy of ligamentum flavum is a significant component in developing lumbar spinal canal stenosis with radiculopathy, in addition to degenerative changes occurring in lumbar vertebrae [19]. Many factors, such as aging, mechanical stress, transforming growth factor-beta, and matrix metalloproteinases, are presumed to be involved in the degeneration of ligamentum flavum [20-22].
We presented a case of a young female presented with manifestations of lumbar spinal canal stenosis following COVID-19 affection with Coronavirus.
The ligamentum flavum thickness is an age-dependent phenomenon. Significant changes in LF thickness were witnessed at the L4-L5 and L5-S1 spinal levels as age increased [23].
Abbas et al. [16] and Altinkaya et al. [24] have found that the ligamentum flavum thickness was significantly more significant at the L4-L5 level than the L3-L4 level in subjects with spinal stenosis. In contrast, at the L5-S1 level, no significant difference was seen between patients with spinal stenosis and those without spinal stenosis. They explained this phenomenon by attributing it to the relative hypermobility of these two segments compared with the L5- S1 segment, which is stabilized by illio-lumbar ligaments and the extensive transverse process of the L5 vertebra. In addition, the articular facets of S1 are more coronally oriented, acting to decrease the shearing stress in that segment.
The mean thickness of ligamentum flavum was assessed in many previous studies in correlation to the age in the population between 20-40 years at the L5-S1 level. Sakamaki [15] found it to be 2.5 mm. Altinkaya [24] measured it as 2.7 mm. Kolte [23] found it to be 3.6 mm.
In the patient studied in this study, the ligamentum flavum hypertrophy was calipered as 4.7 mm in L5-S1 level, which was found to be more than the above studies leading to lumbar spinal canal stenosis manifestations.
The impact of COVID-19 on lipid metabolism was discussed by many researchers [25-28]. Yamada et al. [29] found that hypertrophy of the ligamentum flavum was associated with quantitative and qualitative lipid changes and demonstrated total lipid accumulation. Among different types of phospholipase A2 that target cell membrane phospholipids, there is increasing focus on the inflammatory secretory phospholipase A2 IIA (sPLA2-IIA) associated with the severity of COVID-19. Analysis indicates increased sPLA2-IIA levels together with eicosanoids in the sera of COVID patients.
Lysophospholipids, such as lysophosphatidylcholine, could be metabolized by autotaxin (ATX) and converted to lysophosphatidic acid (LPA). Increased ATX has been found in the serum of patients with COVID-19 [30].
Zhou et al. described that Lysophosphatidic acid concentration in the cerebrospinal fluid of patients with hypertrophied ligamentum flavum was higher than those in subjects with non-hypertrophied ligament, and Lysophosphatidic acid-induced hypertrophy of ligamentum flavum [31].
Conclusion
The ligamentum flavum tends to thicken with increasing age. Statistically significant increases in thickness were observed at the L4-L5 and L5-S1 spinal levels. The thickening of the ligamentum flavum is an age-dependent degenerative change. However, according to many previous studies, subjects in the younger age group (20-40) showed a thickness of <4 mm. We presented a case of a young lady less than 30 with hypertrophied ligamentum flavum at an L5-S1 level of more than 4.5 mm and assumed that this hypertrophy may be due to Covid-19 Coronavirus infection.
Conflict of Interests: The authors now represent that they have no conflict of interest to declare. These authors contributed equally to this manuscript/work.
References
- Costandi S, Chopko B, Mekhail M, Dews T, Mekhail N (2015) Lumbar spinal stenosis: therapeutic options review. Pain Pract. 15(1): 68-81.
- Jirathanathornnukul N, Limthongkul W, Yingsakmongkol W, Singhatanadgige W, Parkpian V, et al. (2016) Increased expression of vascular endothelial growth factor is associated with hypertrophic ligamentum flavum in lumbar spinal canal stenosis. J Investig Med. 64(4): 882-7.
- Sakai Y, Ito S, Hida T, Ito K, Harada A, et al. (2017) Clinical outcome of lumbar spinal stenosis based on new classification according to hypertrophied ligamentum flavum. J Orthop Sci. 22(1): 27-33.
- Szpalski M, Gunzburg R (2003) Lumbar spinal stenosis in the elderly: an overview. Eur Spine J. 12(Suppl 2): S170-S175.
- Park JB, Chang H, Lee JK (2001) Quantitative analysis of transforming growth factor-beta 1 in ligamentum flavum of lumbar spinal stenosis and disc herniation. Spine (Phila Pa 1976). 26(21): E492-5.
- Sairyo K, Biyani A, Goel V, Leaman D, Booth R Jr, et al. (2005) Pathomechanism of ligamentum flavum hypertrophy: a multidisciplinary investigation based on clinical, biomechanical, histologic, and biologic assessments. Spine (Phila Pa 1976). 30(23): 2649-56.
- Fukuyama S, Nakamura T, Ikeda T, Takagi K (1995) The effect of mechanical stress on hypertrophy of the lumbar ligamentum flavum. J Spinal Disord. 8(2): 126-30.
- Kong MH, Morishita Y, He W, Miyazaki M, Zhang H, et al. (2009) Lumbar segmental mobility according to the grade of the disc, the facet joint, the muscle, and the ligament pathology by using kinetic magnetic resonance imaging. Spine (Phila Pa 1976). 34(23): 2537-44.
- Moon HJ, Park YK, Ryu Y, Kim JH, Kwon TH, et al. (2012) The angiogenic capacity from ligamentum flavum subsequent to inflammation: a critical component of the pathomechanism of hypertrophy. Spine (Phila Pa 1976). 37: E147-55.
- Shafaq N, Suzuki A, Terai H, Wakitani S, Nakamura H (2012) Cellularity and cartilage matrix increased in hypertrophied ligamentum flavum: histopathological analysis focusing on the mechanical stress and bone morphogenetic protein signaling. J Spinal Disord Tech. 25: 107-15.
- Viejo-Fuertes D, Liguoro D, Rivel J, Midy D, Guerin J (1998) Morphologic and histologic study of the ligamentum flavum in the thoraco-lumbar region. Surg Radiol Anat. 20(3): 171-6.
- Hayashi K, Suzuki A, Abdullah Ahmadi S, Terai H, Yamada K, et al. (2017) Mechanical stress induces elastic fibre disruption and cartilage matrix increase in ligamentum flavum. Sci Rep. 7: 13092.
- Lakemeier S, Schofer MD, Foltz L, Schmid R, Efe T, et al. (2013) Expression of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and matrix metalloproteinases 1, 3, and 9 in hypertrophied ligamentum flavum. J Spinal Disord Tech. 26(7): 400-6.
- Kosaka H, Sairyo K, Biyani A, Leaman D, Yeasting R, et al. (2007) Pathomechanism of loss of elasticity and hypertrophy of lumbar ligamentum flavum in elderly patients with lumbar spinal canal stenosis. Spine (Phila Pa 1976). 32(25): 2805-11.
- Sakamaki T, Sairyo K, Sakai T, Tamura T, Okada Y, et al. (2009) Measurements of ligamentum flavum thickening at lumbar spine using MRI. Arch Orthop Trauma Surg. 129(10): 1415-9.
- Abbas J, Hamoud K, Masharawi YM, May H, Hay O, et al. (2010) Ligamentum flavum thickness in normal and stenotic lumbar spines. Spine (Phila Pa 1976). 35(12): 1225-30.
- Mattar T, Costa A, Appolonio P, Cesar A (2014) Thickness Of The Ligamentum Flavum Of The Spine And Its Relationship With Disc Degeneration. Coluna/Columna. 13(2): 112-5.
- Fujita N, Sakurai A, Miyamoto A, Michikawa T, Tsuji O, et al. (2019) Lumbar spinal canal stenosis leads to locomotive syndrome in elderly patients. J. Orthop. Sci. 24(1): 19–23.
- Yoshida M, Shima K, Taniguchi Y, Tamaki T, Tanaka T (1992) Hypertrophied ligamentum flavum in lumbar spinal canal stenosis. Spine. 17(11): 1353–1359.
- Safak AA, Is M, Sevinc O, Barut C, Eryoruk N, et al. (2010) The thickness of the ligamentum flavum in relation to age and gender. Clin. Anat. 23(1): 79–83.
- Fukuyama S, Nakamura T, Ikeda T, Takagi K (1995) The effect of mechanical stress on hypertrophy of the lumbar ligamentum flavum. J. Spinal Disorders. 8(2): 126–130.
- Park JB, Kong CG, Suhl KH, Chang ED, Riew KD (2009) The increased expression of matrix metalloproteinases associated with elastin degradation and fibrosis of the ligamentum flavum in patients with lumbar spinal stenosis. Clin. Orthop. Surg. 1(2): 81– 89.
- Kolte V, Khambatta S, Ambiye M (2015) Thickness of the Ligamentum Flavum: Correlation with Age and Its Asymmetry- An Magnetic Resonance Imaging Study. Asian Spine J. 9(2): 245–253.
- Altinkaya N, Yildirim T, Demir S, Alkan O, Sarica FB (2011) Factors associated with the thickness of the ligamentum flavum: is ligamentum flavum thickening due to hypertrophy or buckling? Spine (Phila Pa 1976). 36(16): E1093–E1097.
- Minz MM, Bansal M, Kasliwal RR (2020) Statins and SARS- CoV-2 disease: current concepts and possible benefits. Diabetes Metab Syndr. 14(6): 2063–2067.
- Vahedian-Azimi A, Mohammadi SM, Banach M, Beni FH, Guest PC, et al. (2021) Improved COVID-19 Outcomes following Statin Therapy: An Updated Systematic Review and Meta-analysis. Biomed Res Int. 2021: 1901772.
- Goc A, Niedzwiecki A, Rath M (2021) Polyunsaturated ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry. Sci Rep. 11(1): 5207.
- Surma S, Banach M, Lewek J (2021) COVID-19 and lipids. The role of lipid disorders and statin use in the prognosis of patients with SARS-CoV-2 infection. Lipids Health Dis. 20: 141.
- Yamada T, Horikawa M, Sato T, Kahyo T, Takanashi Y, et al. (2021) Hypertrophy of the ligamentum flavum in lumbar spinal canal stenosis is associated with abnormal accumulation of specific lipids. Scientific Reports. 11(1): 23515.
- Farooqui A , Farooqui T , Sun GY, Lin TN, Teh D, et al. (2023) COVID-19, Blood Lipid Changes, and Thrombosis. Biomedicines. 11(4): 1181.
- Zhou T, Du L, Chen C, Han C, Li X, et al. (2018) Lysophosphatidic acid induces ligamentum flavum hypertrophy through the LPAR1/Akt pathway. Cell Physiol. Biochem. 45(4): 1472–1486.