Prakruti Dash1*, Saurav Nayak2
1Associate Professor, Department of Biochemistry, All India Institute of Medical Sciences, Bhubaneswar, Odisha.
2Senior Resident, Department of Biochemistry, All India Institute of Medical Sciences, Bhubaneswar, Odisha.
*Corresponding Author: Prakruti Dash, Associate Professor, Department of Biochemistry, All India Institute of Medical Sciences, Bhubaneswar, Odisha.
Introduction
In a clinical Biochemistry Laboratory, sometimes samples are received from which serum doesn’t get separated easily due to hyperviscosity of the blood. The average speed of centrifugation and time seems inadequate and usually leads to rejecting samples and asking for fresh ones, resulting in unnecessary delay and prolonged turnaround time. Repeat samples also fail to be helpful if the underlying disease condition is the cause of hyperviscosity [1].
This leaves the laboratory staff in a dilemma. Hence, it is necessary to know the reasons behind the hyperviscosity of specific blood samples, methods to overcome such problems, and proper techniques to extract serum from such samples for test reporting. [2] Hyperviscosity results from any pathologic conditions leading to a rise in the cellular components like erythrocytes, leukocytes, and platelets and even more commonly due to the elevation of specific proteins. [3] Polycythemia vera, leukemia, and thrombocytosis are widely encountered, resulting in Hyperviscosity Syndrome (HVS) that comprises cellular components of blood. [4] Other conditions like Sickle cell disease and spherocytosis due to their deformities in the RBCs can also contribute to HVS. The pathologic rise of acellular components like proteins can either be monoclonal or polyclonal. Monoclonal diseases include Waldenstrom macroglobulinemia (WM), cryoglobulinemia, and Multiple myeloma. Polyclonal gammopathies leading to HVS have Rheumatic conditions such as seropositive rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, and HIV infection. Monoclonal gammopathy, like Waldenstrom macroglobulinemia, is the most common cause of HVS. [5,6] Pentamers of IgM are mainly responsible for hyperviscosity in WM, and more than 30 % of all WM patients develop HVS at some point in their life. Myelomas come next in order of causing highly viscous serum, with most cases having IgA, followed by IgG myelomas at less than 5 % of cases.
Laboratory evidence of high serum viscosity establishes the diagnosis. Viscosity is measured in the unit of centipoise (cp). The viscosity of water is 1 cp. Normal serum viscosity relative to water is 1.4 to 1.8 cp. Symptoms of hyperviscosity can appear with a serum viscosity as low as 3 cp but usually arise when it exceeds 4 to 5 cp. [7]
Further investigations comprise a complete blood count (CBC), coagulation profile, urinalysis, and serum chemical parameters. An elevated albumin-protein gap and significant proteinuria on routine urinalysis suggest an underlying Gammopathy. Serum stasis is usually diagnosed by Rouleaux formation on a peripheral blood smear. Serum stasis can also lead to incomplete serum separation by routine centrifugation associated with the malfunction of laboratory testing equipment and preliminary sample analysis. This should raise suspicion of an underlying increase in serum viscosity.
A hyper viscous blood sample was obtained in the clinical Biochemistry Laboratory of a 44-year-old adult male. The patient was admitted to the medical oncology/Hematology department of AIIMS, Bhubaneswar. With a regular centrifugation speed of 1000 rpm and a time of 5 minutes, absolutely no serum could be extracted. The sample received by the Hospital Biochemistry Laboratory was subjected to high-speed centrifugation with increased time, and serum could be removed. Due to insufficient quantity, it was diluted for analysis, and most of the parameters could be evaluated.
The patient was suspected of suffering from any Gammopathy, as evidenced by the protein–albumin gap. Rouleaux formation was seen in the peripheral blood smear.
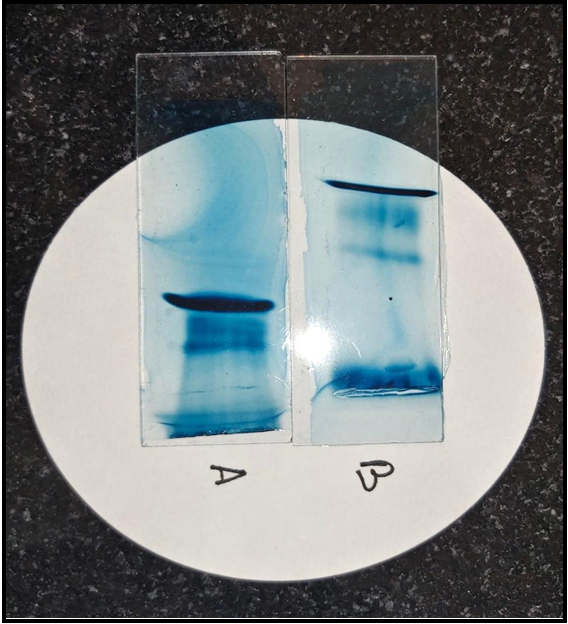
5 ml blood was taken from the patient with written consent and sent for electrophoresis to identify and quantify the paraprotein associated with the hyperviscosity of the blood.
Regular electrophoresis was done, and the M- band was identified in the sample.
Unfortunately, quantitative electrophoresis couldn’t be done due to insufficient samples, and before the patient could be contacted for more examples, he expired.
The presence of abnormal para protein results in hyperviscosity of blood samples and difficulty in separating serum. The increased viscosity of serum relative to water is primarily related to its protein content. Immunoglobulins are relatively large but also linear in shape and hence spin around their longitudinal axis while passing in the serum, thereby increasing serum viscosity more than other serum proteins, which are notably spherical. The type of serum immunoglobulins also contributes and correlates with serum viscosity. IgM, due to its larger size and pentameric structure, can increase the thickness of the serum with a concentration as low as 3 g/dL, and IgM levels of 6 g/dL or higher are strongly linked with quick progression to hyperviscosity resulting in the rapid development of symptomatic HVS. IgA is associated with increased serum viscosity at levels of 6 g/dL or more, attributed to its dimeric form though being smaller in size than IgM. (5, 11). IgG is relatively tiny than both IgM and IgA (180 kDA) and hence can require a higher level, like 10 g/dL, to produce substantial changes in viscosity. The exception is the IgG3 subtype, which due to its predisposition to aggregate in circulation, causes hyperviscosity of serum at low serum levels. [8,9]
Hyperviscosity syndrome due to high levels of para protein is a severe and potentially life-threatening clinical complication. Plasmapheresis remains a necessary interventional procedure for decreasing the symptoms of HVS and reducing serum viscosity. Apart from gammopathies, evidence also shows that initial rituximab therapy is associated with an increase in serum IgM concentrations in 30–70 % of patients resulting in increased viscosity of serum, which must be kept in knowledge while encountering a case of HVS.[10]
The appropriate evaluation of the case and changes in the methodology of handling such samples will benefit the laboratory persons, physicians, and patients. It will create awareness among laboratory technicians, residents, and faculties and help eliminate dilemmas in appropriately handling hyper-viscous blood samples. Adequate knowledge about such conditions can avoid delay due to the rejection of pieces and lead to proper analysis of the model on time.
References
- Gábor K, Péter K, Miklós R, Kálmán T (2008) Plasma Viscosity: A Forgotten Variable. Clinical Hemorheology and Microcirculation. 39(1–4): 243–246.
- Chakraborty S, Chowdhury SR, Krishnan P, Sanyal S, Bhattacharya C, et al. (2014) Improper Serum Separation on Gel Tubes: A Trivial Laboratory Problem or an Indicator of Monoclonal Gammopathy?. Clin Chem Lab Med. 52(12): e275- 8.
- Kwaan HC, Bongu A (1999) The Hyperviscosity Syndromes. Seminars in Thrombosis and Hemostasis. 25(2): 199–208.
- Otto C, Richter WO, Schwandt P (2000) Contribution of Fibrinogen and Lipoproteins to Plasma Viscosity in Hypercholesterolemia and Hypertriglyceridemia: Evaluation by Selective Depletion of Low-Density Lipoproteins or Fibrinogen. Metabolism. 49(6): 810–813.
- Rogers PA, Estes M (2023) Hyperviscosity Syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Gustine JN, Meid K, Dubeau T, Hunter ZR, Xu L, et al. (2017) Serum IGM Level as Predictor of Symptomatic Hyperviscosity in Patients with Waldenström Macroglobulinaemia. British Journal of Haematology. 177(5): 717–725.
- Forchelet D, Béguin S, Sajic T, Bararpour N, Pataky Z, et al. (2018) Separation of Blood Microsamples by Exploiting Sedimentation at the Microscale. Scientific Reports. 8(1): 14101.
- Crawford J, Cox EB, Cohen HJ (1985) Evaluation of hyperviscosity in monoclonal gammopathies. Am J Med. 79(1): 13-22.
- Jin DK, Nowakowski M, Kramer M, Essex DW (2000) Hyperviscosity syndrome secondary to a myeloma-associated IgG(1)kappa paraprotein strongly reactive against the HIV-1 p24 gag antigen. Am J Hematol. 64(3): 210–213.
- Weaver A, Rubinstein S, Cornell RF (2020) Hyperviscosity Syndrome in Paraprotein Secreting Conditions Including Waldenstrom Macroglobulinemia. Front Oncol. 10: 815.