Tung Huynh, PharmD1*, Tiffany Phung, PharmD1, Ke-Qin Hu, MD2#
1Department of Pharmacy, University of California Irvine Health, Orange, CA
2Division of Gastroenterology and Hepatology, University of California Irvine, School of Medicine, Orange, CA
*Corresponding Author: Tung Huynh, PharmD, Department of Pharmacy, University of California Irvine Health, Orange, CA
#Co-Corresponding Author: Ke-Qin Hu, Division of Gastroenterology and Hepatology, University of California Irvine, School of Medicine, Orange, CA
Abstract
Hepatitis C virus treatment with direct-acting antivirals (DAA) has become the standard of care with high sustained virologic response (SVR) rates, shorter duration, and minimal side effects. Data on a retreatment regimen for patients who fail glecaprevir/pibrentasvir are limited. The currently recommended regimen from AASLD for patients with prior glecaprevir/pibrentasvir treatment failure (all genotypes) includes daily glecaprevir/pibrentasvir plus sofosbuvir and weight-based ribavirin for 16 weeks or daily sofosbuvir/velpatasvir/voxilaprevir for 12 weeks. We experienced a unique case of a patient with HIV/HCV co-infection genotype 2b, ESRD on dialysis, who failed 8-week glecaprevir/pibrentasvir monotherapy and was successfully retreated with 12-week glecaprevir/pibrentasvir plus low dose ribavirin without sofosbuvir with close weekly laboratory monitoring and ribavirin dosage adjustment during the treatment. The patient achieved SVR 12, and SVR 24, and was cured of hepatitis C. Our case illustrated a retreatment option for patients with glecaprevir/pibrentasvir treatment failure, the role of ribavirin in the retreatment regimen, and the importance of a multidisciplinary approach with the clinical pharmacist involvement in treating patients with complicated medical conditions to increase successful outcome.
Keywords: Hepatitis C treatment, glecaprevir/pibrentasvir, ribavirin, treatment failure, clinical pharmacist
Introduction
Hepatitis C virus (HCV) infection is a major public health threat and a leading cause of liver-related morbidity and death worldwide, but highly effective therapy may alleviate this burden [1]. Direct-acting antivirals (DAA) have revolutionized the treatment of HCV infection and have become the standard of care with high sustained virologic response (SVR) rates, interferon-free treatment options, shorter duration, and minimal side effects [2]. One remaining challenge is to cure patients who had a virologic failure with prior HCV treatments, especially those containing NS5A inhibitors [3]. The retreatment options in this population are limited which include glecaprevir/pibrentasvir, a fixed-dose combination of glecaprevir, an HCV NS3/4A protease inhibitor and pibrentasvir, an HCV NS5A inhibitor [4] and sofosbuvir/velpatasvir/voxilaprevir, a fixed-dose combination of sofosbuvir, an HCV nucleotide analog NS5B polymerase inhibitor, velpatasvir, an HCV NS5A inhibitor, and voxilaprevir, an HCV NS3/4A protease inhibitor [5]. The retreatment of DAA-experienced patients is an important step to achieve the World Health Organization (WHO) goal to eliminate Hepatitis C by 2030 [6].
Among the most commonly prescribed DAA agents in the United States of America is glecaprevir/pibrentasvir. The pangenotypic combination of glecaprevir/pibrentasvir was approved by Food and Drug Administration (FDA) in 2017 for treatment of HCV genotype 1 to 6 infection without cirrhosis or with compensated cirrhosis (Child-Pugh A) and retreatment of HCV genotype 1 in patients who previously have been treated with a regimen containing NS5A inhibitor or NS3/4A protease inhibitor and retreatment of HCV genotype 1 to 6 infection in patients with prior treatment experience with a regimen containing interferon, ribavirin, and/or sofosbuvir but no-prior treatment experience with HCV NS3/4A PI or NS5A inhibitor [4].
In clinical trials, treatment with glecaprevir/pibrentasvir yielded ≥ 95 % SVR at post-treatment week 12 (SVR 12) across all HCV genotypes, including patients with DAA experience with < 1 % virologic failure rates [7-9]. Studies have shown to increase the success of retreatment, adding ribavirin and a DAA with the non- overlapping mechanism of action to an existing DAA regimen may be beneficial to increase the SVR rates [10,11].
Wyles et al (MAGELLAN-3) study of 12 or 16-weeks combination of glecaprevir/pibrentasvir, sofosbuvir, and ribavirin therapy in HCV infected patients with genotype 1a, 1b, 2a, 3a, and 3b who failed glecaprevir/pibrentasvir monotherapy showed that overall, 96 % of these patients achieved SVR12 [12].
Pearlman et al study of retreatment with 12 weeks of sofosbuvir/velpatasvir/voxilaprevir in HCV infected patients with genotype 1a and 3a who failed glecaprevir/pibrentasvir showed that overall, 94 % of these patients achieved SVR12 [13].
The currently recommended regimen from the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) for patients with prior glecaprevir/pibrentasvir treatment failure (all genotypes) with or without compensated cirrhosis includes glecaprevir/pibrentasvir plus sofosbuvir and weight-based ribavirin for 16 weeks or sofosbuvir/velpatasvir/voxilaprevir for 12 weeks (addition of weight- based ribavirin is recommended for patients with compensated cirrhosis) [14,15].
We experienced a unique case of a patient with HIV/HCV co- infection genotype 2b, end-stage renal disease (ESRD) on dialysis, who failed 8-week glecaprevir/pibrentasvir monotherapy and was successfully retreated with 12-week glecaprevir/pibrentasvir plus low dose ribavirin without sofosbuvir with close weekly laboratory monitoring and the clinical pharmacist involvement during the treatment. The patient achieved SVR 12, and SVR 24, and was cured of hepatitis C.
Case Presentation
A 61-year-old male with multiple complicating co-morbidities including past medical history of the human immunodeficiency virus (HIV) on highly active antiretroviral therapy (HAART), ESRD on dialysis, hypertension, pre-diabetes, coronary artery disease (CAD) status post coronary stent in June 2016, and chronic hepatitis C genotype 2b infection. The patient was diagnosed with HIV in 1990 and his HIV infection was well controlled on a HAART regimen with a stable CD4 count of around 200 cells/mm3 and undetectable HIV viral load with no complications. His HIV regimen included abacavir, darunavir, raltegravir, and ritonavir.
The patient was diagnosed with HCV around 1997, genotype 2b. He might have contracted the virus from a blood transfusion that he received around 1997. He had previously been treated twice for hepatitis C, but he had relapsed with both treatments. He was first treated around 2005 with peg-interferon alfa-2a and ribavirin for 8 months. His HCV viral load was undetectable initially post-treatment, and subsequently, his HCV viral load returned. The patient was listed for a kidney transplant in early 2016. Due to advanced stage 3 fibrosis on elastography and liver biopsy and the risk of accelerated progression in HCV/HIV co-infection, the patient needed to have retreated before kidney transplant. During that time, the only treatment option for the patient was glecaprevir/pibrentasvir which was approved for HCV genotype 2 infected patients with interferon and ribavirin treatment-experienced and ESRD. The patient was retreated with an 8-week glecaprevir/pibrentasvir monotherapy on 10/28/2017 and continued with the current HIV regimen. His baseline laboratories included HCV RNA of 648,950 IU/mL (5.81 log10), genotype 2b, ALT 32 IU/L, platelets 83 x103/µL, and hemoglobin 11.3 g/dL. He completed the treatment with no sign of drug-drug interaction and his HIV condition was stable during the treatment. He had undetectable viral load at treatment week 4 and week 8, but he had detectable viral load at 8 weeks after completing the treatment. He was in the process of being referred and evaluated to receive a kidney transplant in late 2018. Due to the patient’s elevated risk of progression of liver fibrosis with multiple complicated co-morbidities, the patient was referred to our hepatology clinic for HCV retreatment before kidney transplantation.
The patient’s liver biopsy on 09/2017 revealed he had stage 3 fibrosis. He also had compensated cirrhosis, with a MELD (MELD-Na) score of 21, Child-Pugh A, thrombocytopenia, evidence of esophageal varices, and portal hypertensive gastropathy (PHG) on Esophagogastroduodenoscopy (EGD) on 07/2018. In addition, the patient had relapsed twice for HCV treatments and one time with DAA containing NS5A inhibitor. The decision was made to retreat the patient a third time before the kidney transplantation. At this time, sofosbuvir and sofosbuvir/velpatasvir/voxilaprevir were not approved or recommended for patients with severe renal impairment or with ESRD on dialysis and only glecaprevir/pibrentasvir was approved to use in this population. The only treatment option for this patient was the 12-week course of glecaprevir/pibrentasvir plus ribavirin. Due to his ESRD, we expected he would have a very low tolerance to ribavirin and a high risk of anemia, and we would plan to start at a very low dose of ribavirin and kept the same HAART regimen as prior with glecaprevir/pibrentasvir treatment. Our multidiscipline team of hepatologists and the clinical pharmacist decided to retreat the patient with 12-week glecaprevir/pibrentasvir and dosed ribavirin of 200 mg daily without sofosbuvir but with close monitoring. The patient’s baseline laboratories included HCV RNA 1,453,880 IU/ml (6.16 log10), genotype 2b, ALT 83 IU/L, platelets 96 x103/µL, and hemoglobin 11.1 g/dL.
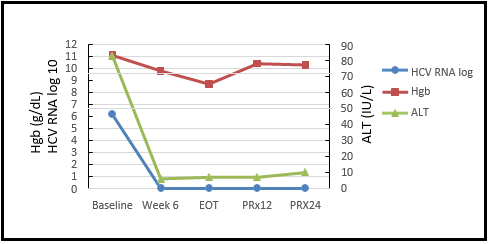
The patient started HCV retreatment and agreed to have weekly laboratory monitoring of hemoglobin levels. As shown in Table 1, his initial hemoglobin level was 11.1 g/dL. During the treatment, the hemoglobin was dropped, and ribavirin dosage was adjusted timely from 200mg daily at the start of treatment to 200mg two times a week at treatment week 1 and 200mg once a week at treatment week 11 through the end of treatment. The patient tolerated adjusted ribavirin doses and completed the treatment without any adverse events. His HIV infection was controlled and stable during the retreatment. The patient achieved SVR 12 and SVR 24 with ALT 7 IU/L and 10 IU/L, platelets 90 x103/µL and 123 x103/µL, hemoglobin 10.4 g/dL and 10.3 g/dL at post-treatment week 12 and week 24, respectively, and undetectable HCV RNA at both times (Figure 1).
Table 1: Weekly Hemoglobin Laboratory Monitoring, Dynamic Changes of Biochemical and Virological Responses, and RBV Dose Adjustment during Treatment
|
|
Baseline |
Start Rx |
Day 2 |
Wk 1 |
Wk 2 |
Wk 4 |
Wk 6 |
Wk 8 |
Wk 11 |
Wk 12 (EOT) |
PRx Wk 2 |
PRx Wk12 |
PRx Wk24 |
|
Hgb (g/dL) |
11.1 |
10.8 |
11.2 |
10.3 |
10.6 |
10.3 |
9.8 |
9.4 |
8.5 |
8.7 |
8.9 |
10.4 |
10.3 |
|
Platelets (103/µL) |
96 |
99 |
86 |
|
84 |
89 |
90 |
|
100 |
106 |
|
90 |
123 |
|
ALT (IU/L) |
83 |
|
|
|
9 |
|
6 |
|
|
7 |
|
7 |
10 |
|
ALP (IU/L) |
57 |
|
|
|
43 |
|
44 |
|
|
54 |
|
51 |
72 |
|
HCV RNA (IU/mL) |
1,453,880 |
|
|
|
|
0* |
0 |
|
|
0 |
|
0 |
0 |
|
RBV 200mg dosing |
|
QD |
QOD |
BIW |
BIW |
BIW |
BIW |
BIW |
QWk |
QWk |
|
|
|
|
Abbreviations: Rx, treatment; Hgb, hemoglobin; Wk, week; EOT, end of treatment; PRx, post-treatment; AST, aspartate aminotransferase; ALP, alkaline phosphatase; RBV, ribavirin; QD, once a day; QOD, every other day; BIW, two times a week; QWk, once a week * 0: undetectable HCV RNA |
|||||||||||||
Figure 1: Dynamic Changes of HCV RNA, Hemoglobin and Alanine Aminotransferase before, during, and after Glecaprevir/Pibrentasvir Treatment
The HCV RNA was rapidly declined to undetectable at treatment week 6 and remained undetectable till post-treatment week 24, the patient achieved sustained virologic response SVR12 and SVR24. The ALT was also rapidly improved at treatment week 6 and remained normalized till post-treatment week 24. Hemoglobin level was dropped during the treatment with ribavirin and improved after stopping ribavirin.
Abbreviations: Hgb, hemoglobin; ALT, alanine aminotransferase; EOT, end of treatment; PRx, post-treatment week
Discussion
This case was very unique with the off-label use of glecaprevir/pibrentasvir in HCV genotype 2b treatment failure and different treatment options from AASLD recommendation. The patient was successfully retreated with 12-week glecaprevir/pibrentasvir with low dose ribavirin without sofosbuvir after failed 8-week glecaprevir/pibrentasvir monotherapy. Since the patient failed HCV treatment twice and one time with DAA, adding ribavirin may be beneficial for achieving SVR. The exact mechanism by which ribavirin helps to cure hepatitis C is poorly understood, however, the use of ribavirin in combination with DAA may still be necessary and continue to be part of combination therapy with DAA [16]. Lu et al study had demonstrated that the addition of ribavirin in the treatment regimen significantly increased the likelihood of SVR among patients with prior DAA treatment failure [17]. In this case, the patient’s ESRD may increase the risk of ribavirin adverse effects. The patient did not start with weight-based ribavirin dosing but only with renally dosed and weekly laboratory monitoring with dosage adjustment according to the patient’s tolerance and laboratory results. This case illustrated that the addition of low-dose ribavirin to the glecaprevir/pibrentasvir regimen might provide additional coverage to help eradicate the virus and to prevent viral relapse in ESRD patients with treatment failure and re-demonstrated the role and benefits of ribavirin in HCV retreatment regimen even with a lower dose.
This is an unusual case with the off-label use of glecaprevir/pibrentasvir in HCV retreatment with a 12-week treatment duration without sofosbuvir. Studies have shown that increasing treatment duration improved the success rate. In patients with 8-week treatment failure, Lu et al study demonstrated that increasing treatment duration improved SVR rates with each additional month (4 weeks) of treatment reduced the risk of non-SVR by 50% or each month increase in DAA treatment duration reduced the rate of treatment failure by 50 % [17]. Wyles et al study demonstrated that patients who had a virologic failure with glecaprevir/pibrentasvir can be retreated successfully with glecaprevir/pibrentasvir with sofosbuvir and ribavirin for 12 or 16 weeks [12]. The present case demonstrated that in patients with DAA failure adding ribavirin with a longer duration may increase the DAA retreatment outcome. Naganuma et al study reported a case of 78- year-old Japanese DAA-naïve non-cirrhotic patient genotype 2a who showed virologic relapse at post-treatment week 13 following 8-week glecaprevir/pibrentasvir therapy and achieved SVR 12 after prolonged 12-week glecaprevir/pibrentasvir therapy [18]. Compared to the reported case from the Naganuma study, the present case was consistent and similar with prolonged 12-week retreatment after 8- week treatment failure and achieving SVR 12, but it was different with the addition of low dose ribavirin, and patient having more complicated co-morbidities including HIV/HCV co-infection, HCV treatment failure twice, ESRD, CAD, and stage 3 fibrosis. This case report suggested that a longer duration of at least 12-week duration may be needed and may have an effect on improving the SVR rate in patients with complicated medical conditions. In the real world, there is limited information on the efficacy of retreatment with DAA regimen in failures of prior DAA treatment. This case illustrated another retreatment option and support the use of glecaprevir/pibrentasvir with ribavirin without sofosbuvir for at least 12-week duration as a salvage regimen after treatment failure of glecaprevir/pibrentasvir monotherapy in patients who could not take sofosbuvir or sofosbuvir/velpatasvir/voxilaprevir.
Hemolytic anemia is one of the side effects of ribavirin that had prohibited many patients from completing the therapy, therefore, closely monitoring is highly recommended, especially in patients with high risk and complicated medical conditions. In this case, it should be noticed that there was strong interaction and participation between the patient, hepatologist, and pharmacist. The patient agreed to have a weekly blood test. The laboratory monitoring and the dosage adjustment were made and followed up closely by the clinical pharmacist and hepatologist to prevent any adverse events. During the clinic visit, the patient’s tolerability, medication compliance, and treatment responses were assessed by the clinical pharmacist through patient consultation, medications review, and laboratory monitoring. The pharmacist reviewed the laboratory results and discussed with the hepatologist about the treatment plan. In the present case, the pharmacist made the ribavirin dosage adjustment timely from 200 mg daily to 200 mg once a week to prevent further hemoglobin reduction and side effects. With such efforts from the multidisciplinary team and the patient, the DAA treatment course was not interrupted, and there were no unexpected adverse events during the retreatment. The patient achieved SVR 12 and was cured of hepatitis C. This case demonstrated the effectiveness of strong participation between providers and patients, and the clinical pharmacist involvement in taking care of patients with complicated co-morbidities to improve the SVR rate and the treatment outcome.
In this case, sofosbuvir/velpatasvir/voxilaprevir could be another option for the third retreatment, however, during that time it could not be used in this patient because it was not approved to use with no dosage recommendations in patients with severe renal impairment and ESRD. In November 2021, the United States of America FDA approved the changes to the product label for sofosbuvir/velpatasvir/voxilaprevir to include new efficacy and safety data for adults with hepatitis C and severe renal impairment, including a patient who requires dialysis based on pharmacokinetic data obtained from studies involving HCV-infected patients with ESRD requiring dialysis. Gilead company updated sofosbuvir/velpatasvir/voxilaprevir prescribing information with no dosage adjustment is recommended in patients with any degrees of renal impairment including patients on dialysis [5]. This will provide more options to treat HCV infections in this population and help to move closer toward achieving the WHO goal of elimination of HCV infection by 2030.
Conclusions
To our knowledge, this is one unique case of successful retreatment of 8-week glecaprevir/pibrentasvir monotherapy failure patient with 12-week glecaprevir/pibrentasvir without sofosbuvir and low dose ribavirin in HIV/HCV co-infected and ESRD patients. It showed that prolonged treatment duration and addition of ribavirin may be needed and may increase the success rates to achieve SVR in treatment failure patients and the effectiveness of the multidisciplinary approach with the clinical pharmacist involvement in treating patients with complicated medical conditions. Our experience not only enriches our knowledge but also provides an additional treatment option for patients with glecaprevir/pibrentasvir failure. We assume that this unique case may apply to other patients; however, more study with a larger sample size is needed to confirm the finding of our case report.
Patient Consent: Obtained
Source of Funding: None
Disclosures: Tung Huynh and Tiffany Phung have nothing to disclose. Ke-Qin Hu is on the speaker bureau for Gilead Sciences.
Author Contribution: TH, writing, draft preparation, final writing, and editing. TP, writing, and draft preparation. KQH, final writing, and editing.
Conflicts of Interest: The authors declare no conflicts of interest.
References
- Cooke GS, Andrieux-Meyer I, Applegate TL, Atun R, Burry JR, et al. (2019) Accelerating the elimination of viral hepatitis: A lancet gastroenterology & hepatology commission. Lancet Gastroenterol Hepatol. 4(2): 135-84.
- Hezode C (2018) Treatment of hepatitis C: Results in real life. Liver Int. 38(suppl 1): 21-27.
- Sarrazin C (2016) The importance of resistance to direct-acting antiviral drugs in HCV infection in clinical practice. J Hepatol. 64(2): 486-504.
- AbbVie Inc. Mavyret (glecaprevir and pibrentasvir) package insert, 2020.
- Gilead Sciences, Inc. Vosevi (sofosbuvir, velpatasvir, and voxilaprevir) package insert, 2020.
- World Health Organization. (2016) Combating hepatitis B and C to reach elimination by 2030.
- Puoti M, Foster GR, Wang S, Mutimer D, Gane E, et al. (2018) High SVR12 with 8-week and 12-week glecaprevir/pibrentasvir therapy: an integrated analysis of HCV genotype 1-6 patients without cirrhosis. J Hepatol. 69(2): 293-300.
- Forns X, Lee SS, Valdes J, Lens S, Ghalib R, et al. (2017) Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infections in adults with compensated cirrhosis (EXPEDITION-I): a single-arm, open-label, multicenter phase 3 trial. Lancet Infect Dis. 17(10): 1062-1068.
- Poordad F, Pol S, Asatryan A, Buti M, Shaw D, et al. (2018) Glecaprevir/Pibrentasvir in patients with hepatitis C virus genotype 1 or 4 and past direct-acting antiviral treatment failure. Hepatology. 67(4): 1253-1260.
- Wyles D, Pockros P, Morelli G, Younes Z, Svarovskaia E, et al. (2015) Ledipasvir-sofosbuvir plus ribavirin for patients with genotype 1 hepatitis C virus previously treated in clinical trials of sofosbuvir regimens. Hepatology. 61(6): 1793-1797.
- Lawitz E, Poordad F, Wells J, Hyland RH, Yang Y, et al. (2017) Sofosbuvir-velpatasvir-voxilaprevir with or without ribavirin in direct-acting antiviral-experienced patients with genotype 1 hepatitis C virus. Hepatology. 65(6): 1803-1809.
- Wyles D, Weiland O, Yao B, Weilert F, Dufour JF, et al. (2019) Retreatment of patients who failed glecaprevir/pibrentasvir treatment for hepatitis C virus infection. J Hepatol. 70(5): 1019- 1023.
- Pearlman B, Perrys M, Hinds A (2019) Sofosbuvir/velpatasvir/voxilaprevir for previous treatment failures with glecaprevir/pibrentasvir in chronic hepatitis C infection. Am J Gastroenterol. 114(9): 1550-1552.
- American Association for the Study of Liver Diseases - Infectious Diseases Society of America (AASLD-IDSA). Glecaprevir/Pibrentasvir Treatment Failure (All Genotypes).
- European Association for the Study of the Liver (EASL). (2020) EASL recommendations on treatment of hepatitis C: Final update of the series. Journal of Hepatology. 73(5): 1170 -1218.
- Koh C, Liang TJ (2014) What is the future of ribavirin therapy for hepatitis C? Antiviral Research. 104: 34-39.
- Lu M, Wu KH, Li J, Moorman AC, Spradling PR, et al. (2019) Adjuvant ribavirin and longer direct-acting antiviral treatment duration improve sustained virologic response among hepatitis C patients at risk of treatment failure. J Viral Hepat. 26(10): 1210-1217.
- Naganuma A, Sato K, Fukuchi T, Namikawa M, Kakizaki S, et al. (2019) Successful prolonged treatment of glecaprevir/pibrentasvir for chronic hepatitis C patient with treatment failure after 8-week therapy: a case report. Clinical Journal of Gastroenterology. 12(6): 592-597.