A B M Nasibul Alam*, Natalia P. Ballestas, Tatjana Blazin, Dhruvil Prajapati, Linha (Lina) M. Mohammed, Meera Dhavale, Mohamed K. Abdelaal, Jihan A. Mostafa
California Institute of Behavioral Neurosciences & Psychology, Fairfield, USA
*Corresponding Author: A B M Nasibul Alam, California Institute of Behavioral Neurosciences & Psychology, Fairfield, USA
Abstract
In the late antepartum and the early postpartum period, peripartum cardiomyopathy (PPCM) is an important etiology of heart failure. A disease of increasing severity, PPCM has substantial heterogeneity in its regional and ethnic distribution. Besides, findings of multiple studies conducted in the last two decades have demonstrated that a subset of PPCM might comprise a portion of the Familial Dilated Cardiomyopathy (DCM) syndrome.
A research article published in 2008 demonstrated that 16 kDa prolactin is involved in the pathogenesis of PPCM. Consequently, multiple studies were conducted to assess the efficacy of bromocriptine, a prolactin release inhibitor drug as a disease-specific therapeutic agent for PPCM. Analysis of these studies in our article showed that bromocriptine improves clinical and echocardiographic outcomes in PPCM patients. However, it is important to mention that there are some valid concerns regarding the acceptance of bromocriptine as a therapeutic option in PPCM due to certain deficiencies of the available data including a small number of studies, and the studies themselves having various limitations, the lack of diversity in the study population is a crucial one. In addition, there are concerns regarding the prothrombotic adverse effects of bromocriptine in PPCM patients. Hence, it has been recommended to use additional prophylactic anticoagulation along with bromocriptine. The available data suggest that prophylactic anticoagulation might be effective in reducing or even eliminating the risk of thrombosis associated with the use of bromocriptine in PPCM.
Therefore, we recommend conducting more studies, especially large-scale international studies, with diverse study populations before reaching any conclusion regarding the efficacy and safety of bromocriptine in PPCM. The already available pieces of information that we have summarized and reviewed in our article would assist in planning and designing such studies.
Introduction
In 1971, Demakis et al. [1] first defined peripartum cardiomyopathy (PPCM) based on 3 specific criteria. One of these criteria limited the diagnosis of PPCM to the period extending from the last gestational month to the first five months in the postpartum period. Since then, the definition of PPCM has evolved, and been expanded.
A recent definition of PPCM given by a working group on PPCM of European Society of Cardiology [2] defines PPCM as “ idiopathic cardiomyopathy presenting with heart failure secondary to left ventricular systolic dysfunction towards the end of pregnancy or in the months following delivery, where no other cause of heart failure is found. It is a disease of exclusion. The left ventricle may not be dilated but the ejection fraction is nearly always reduced below 45 %.”
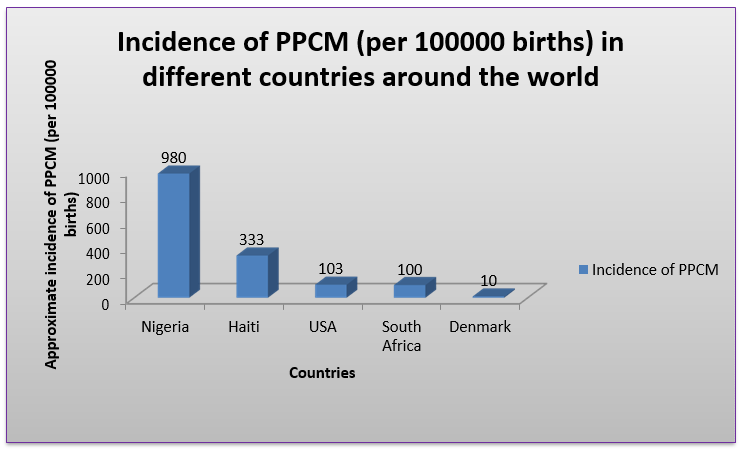
The incidence of PPCM is on the rise [3, 4], and it greatly varies from country to country (figure 1) [3,5-8]. For example, in Nigeria, the incidence of PPCM was found to be 1 in 102 deliveries [5] whereas, in Denmark, the incidence was estimated as 1 in 10,149 deliveries [8]. A major factor contributing to this wide variation in incidence rates may be attributed to the fact that African ancestry is considered to be an important risk factor for PPCM [3,9]. Moreover, the prognosis is worse in women of African origin in comparison to other ethnic groups [10]. In addition, genetic susceptibility may contribute to a higher incidence of PPCM in certain regions compared to the rest of the world [11]. Again, it has also been suggested that genetic predisposition may be a poor prognostic factor for PPCM [12,28,45].
Figure 1: Incidence of PPCM (per 100000 births) in different countries around the world
The conventional medications used to treat PPCM include common medications used to treat other causes of diastolic heart failure. They are loop diuretics, beta-blockers, hydralazine/isosorbide dinitrate, digoxin, Angiotensin-Converting Enzyme-1 inhibitors (ACE-1 inhibitors), Angiotensin Receptor Blockers (ARBs), and anticoagulants if a left ventricular (LV) thrombus is formed or there is a significant risk of LV thrombus formation(LV ejection fraction < 35 %). But certain drugs (i.e., ACE-inhibitors, ARBs, anticoagulant warfarin) are recommended to avoid in pregnancy due to their teratogenic effects [2, 14].
A groundbreaking study by Hilfiker-Kleiner et al. [13] brought forth the prospect that a prolactin inhibitor drug like bromocriptine might be a disease-specific therapeutic option for PPCM. Published in 2008, this study demonstrated that oxidative stress resulting in the generation of cardiotoxic 16kD form of the lactation-promoting hormone prolactin is instrumental in the pathogenesis of PPCM. This finding inspired the conduction of multiple studies in subsequent years to assess bromocriptine as a therapeutic agent for PPCM. The findings of these studies have shown promising outcomes with bromocriptine use in PPCM patients. Recently, the European Society of Cardiology (ESC) guidelines have recommended that a short-term and a long-term regimen of bromocriptine based on the severity of symptoms and Left Ventricular Ejection Fraction (LVEF) may be considered in PPCM [15]. On the other hand, the American College of Cardiology (ACC) still does not recommend bromocriptine as a treatment option for PPCM in its guidelines [14].
Certain limitations of the available data (i.e., small number of studies in only a few countries in the world) maybe responsible for the fact that bromocriptine is not universally recommended as a treatment option for PPCM. Again, there have also been concerns regarding the safety of bromocriptine use in PPCM [33]. In addition, due to the multifactorial etiology of PPCM, it has been implied that bromocriptine treatment may not be equally effective in all PPCM patients [31]. However, we need more studies before reaching a conclusion regarding this implication. Moreover, more data are required regarding influences, if any, of ethnicity and genetic predisposition on the therapeutic outcome of bromocriptine use in PPCM.
Therefore, the objective of this review is to summarize the ongoing state of knowledge and highlight the unanswered questions along with the future prospect of this relatively new concept based on the available data and expert opinions. We also plan to discuss the pathophysiology of PPCM, particularly the role of prolactin, since this pathophysiological finding laid the groundwork for ongoing discussions and debate on this topic.
Discussion
PPCM Pathophysiology
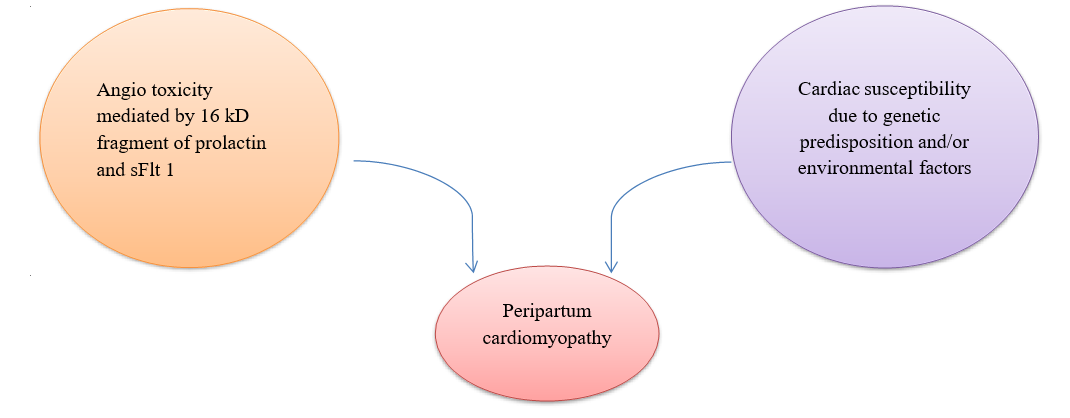
Multiple factors are implied to be responsible for the pathogenesis of PPCM. In the last two decades, various theories have been put forward to comprehensively explain the complex interplay among different etiological factors responsible for PPCM. The two-hit model of PPCM pathogenesis is one of these theories.
According to this model, it is hypothesized that the late gestational antiangiogenic hormonal environment (1st hit) causes PPCM in an already susceptible woman (2nd hit) [19]. (Figure 2)
Figure 2: The two-hit hypothesis of Peripartum Cardiomyopathy. sFlt-1 = soluble Fms-like tyrosine 1
Explanation of the First Hit
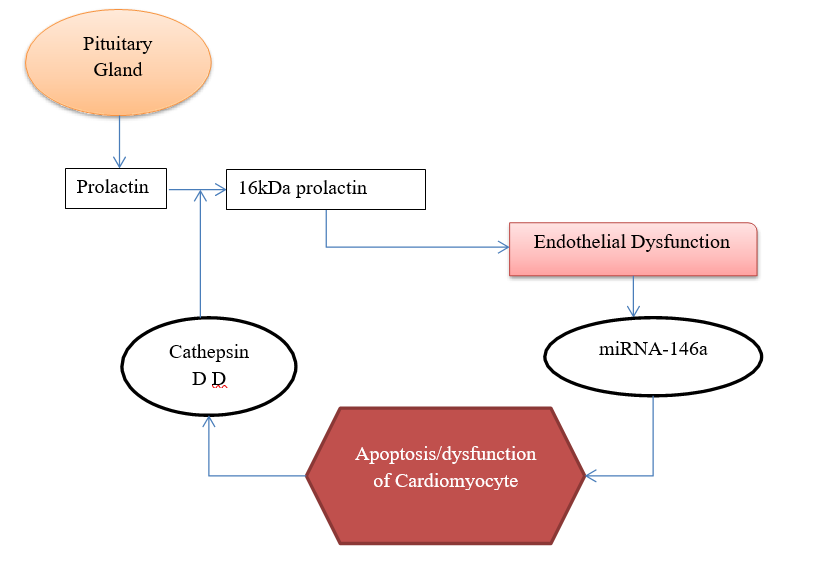
Hilfiker-Kleiner et al [12] demonstrated that excess secretion of Cathepsin D (CD) from cardiac myocyte in STAT knockout (KO) mice lead to cleavage of prolactin into a 16kD fragment. Endothelium exposed to this 16kD fragment induces expression of microRNA146a, which in turn is taken up by the adjacent cardiomyocytes, and subsequently, anti-apoptotic genes are suppressed in these cardiomyocytes [28]. As a result, increased apoptosis occurs in cardiomyocytes leading to PPCM [13, 19]. This pathophysiology is illustrated in Figure 3.
Figure 3: Pathophysiology of Prolactin mediated cardiomyocyte apoptosis/dysfunction.
In another study [20], it was demonstrated that PGC-1 alpha, a transcription coactivator of energy metabolism in various cell types, leads to Angio toxicity in the late gestational period. According to this study, two pathways are implied to be involved in the development of such Angio toxicity. One is the activation of the aforementioned antiangiogenic 16 kD prolactin mediated pathway, and the other one is the loss of the proangiogenic Vascular Endothelial Growth Factor (VEGF) mediated pathway. The effects of loss of VEGF are accentuated by increased placental secretion of soluble Fms-like tyrosine 1 (sFlt-1) in late gestation. Under normal circumstances, VEGF protects against the cardiotoxic effects of sFlt-1. But the absence of PGC-1 alpha significantly reduces the production of VEGF resulting in the impediment of its cardioprotective activity. Interestingly, it was noticed in this study that the recovery of PGC-1 alpha cardiac KO mice was achieved with the combination of bromocriptine and VEGF, not bromocriptine alone.
Bello et al. [19] suggested that genetic predisposition and environmental factors such as viral myocarditis possibly comprise the 2nd hit. It has also been speculated that hypertension, nutritional factors (i.e., selenium deficiency, or autoimmunity can play a role in further increasing the susceptibility. Therefore, a ‘multifactorial susceptibility’ theory of PPCM has been implied, but more evidence is required to support it [21].
Genetic Predisposition as an Etiological and Prognostic Factor
Multiple studies have found very similar genetic mutations in a portion of cases of PPCM and familial Dilated Cardiomyopathy (DCM). Especially, various mutations in TTN (a gene that encodes for the protein titin) have been reported in a good number of cases of both familial DCM and PPCM. These pieces of evidence suggest that at least one subset of PPCM is part of the spectrum of familial Dilated Cardiomyopathy (DCM) [12,22,23,45].
Moreover, it has been noticed in several studies that PPCM cases with a possible genetic predisposition have a worse prognosis compared to cases with no genetic predisposition [12,28,45]. In fact, a prospective cohort study conducted by Haghikia et al. [28] found that patients with a positive family history of PPCM were three times more likely to receive heart transplantation.
These findings make it relevant to consider that PPCM cases with a genetic predisposition may respond differently to various treatment options including bromocriptine. However, more studies should be performed to justify these implications regarding genetic susceptibility in PPCM, and its effect on treatment outcome.
Role of Bromocriptine in the Management of PPCM
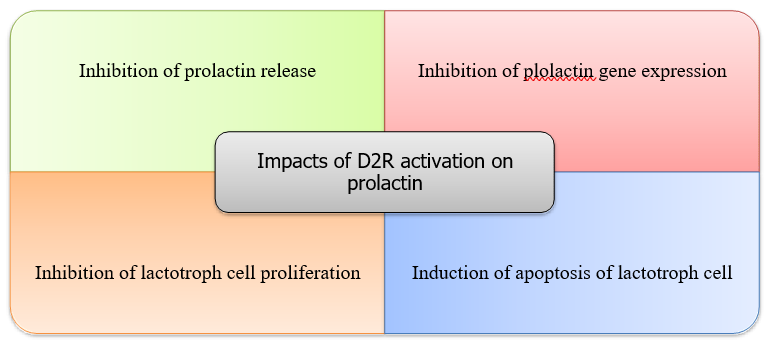
Benefit of Bromocriptine as a therapeutic agent in PPCM Bromocriptine is a semisynthetic ergot derivative. It is a D2 receptor (D2R) agonist and D1 receptor (D1) antagonist. It exerts its prolactin inhibitory function by binding with and activating D2R via several complex and intricate pathways. Figure 4 gives a glimpse into the array of effects D2R activation has on prolactin secretion and synthesis [24, 25, 40].
Figure 4: Impacts of Type 2 Dopamine Receptor (D2R) activation on prolactin
In lactotrophs, stimulation of D2R decreases intracellular calcium ion (Ca2+), which in turn reduces the delivery of prolactin from the anterior pituitary since Ca2+ assists in the fusion of synaptic vesicles with the cell membrane which is essential for exocytosis of prolactin [25, 26].
There have been reports on bromocriptine induced apoptosis in the pituitary cells, especially via p38 mitogen-activated protein (MAP) kinase activation [41]. Bromocriptine has also been found to induce apoptosis by suppressing Bcl-2 expression in pituitary cells [42]. Some studies suggest that bromocriptine induces proptosis [43] and autophagic cell death (ACD) [44] as well. However, more evidence is required to reach any definitive conclusion regarding these mechanisms.
In addition to prolactin inhibition, bromocriptine has a direct beneficial impact on hemodynamic parameters as well. G S Francis et al. [37] demonstrated that bromocriptine decreases mean blood pressure, systemic vascular resistance, and left ventricular filling pressure, and increases stroke volume index. The direct vasodilatory effect of bromocriptine via vascular dopaminergic receptors and/or the reduction in norepinephrine level due to its use may be responsible for such changes in hemodynamic parameters. Most notably, all these changes have a beneficial outcome in congestive heart failure.
Combinations and Complications:
A prospective study conducted by Sliwa K et al. [27] to assess the benefit of bromocriptine use in PPCM had a study population of 20 who were randomized into two groups, each containing 10. Both groups were homogenous in terms of the baseline characteristics. One group received standard heart failure therapy alone whereas the other group received additional bromocriptine therapy. Patients receiving additional bromocriptine therapy showed a much better prognosis with nine of them surviving during the six-month follow-up period compared to those not receiving bromocriptine (four of them died during the six-month follow-up period). Moreover, greater improvement in the LVEF was noticed in the group receiving bromocriptine compared to the control group (27 % to 58 %; P=0.012). (Table 1). However, this study had notable limitations. For example, it was an open-label, single-center study in which the study population was small. Moreover, the mortality rate in the control arm of the study was higher than the mortality rate of PPCM demonstrated in other studies.
Table 2: A summary of the characteristics of important studies analyzed in this literature review. LVEF = Left Ventricular Ejection Fraction, BRC group = the group that received bromocriptine, non-BRC group = the group that did not receive bromocriptine, 1W group = the group that received bromocriptine for 1 week, 8W group = the group that received bromocriptine for 8 weeks
|
Author |
Study design |
Study population (n) |
Death/ lost due to follow-up or other causes |
Baseline LVEF |
LVEF after treatment |
Follow-up duration |
Thromboembolism (n) |
|
Sliwa et al. [27] |
RCT |
20 (BRC group: 10, Non-BRC group: 10)
|
BRC group: 1 |
27 ± 8%
|
BRC group: 58 ± 11%
|
6 months |
0 |
|
Non-BRC group: 4
|
Non-BRC group: 36 ± 11%
|
||||||
|
Haghikia et al. [28] |
Cohort (P)
|
115
|
2 deaths, 19 lost to follow up |
27 ± 9%
|
47 ± 19% in all patients
|
6 ± 3 months |
None reported |
|
BRC group: 92% demonstrated LVEF improvement
|
|||||||
|
Non-BRC group: 72 % Demonstrated LVEF improvement. |
|||||||
|
Yameogo et al. [29] |
RCT |
96 (BRC group: 48, Non-BRC group: 48) |
Death: BRC group: 8
|
BRC group: 37.2 ± 6.6 %
|
BRC group: 53.9 ± 4.1% |
12 months |
0 |
|
Death: Non-BRC group: 14
|
Non-BRC group: 37.5 ± 4.8 % |
Non-BRC group: 45.9 ± 5.9 % ( at 12 months follow up)
|
|||||
|
Hilfiker-Kleiner and Haghikia et al. [30] |
RCT |
63 (1W group: 32, 8W group: 31) |
No death. 6 patients lost due to various causes. |
1W group: 28 ± 10%
|
1W group: 49 ± 12 %
|
6 months |
2 venous embolisms and 1 peripheral artery occlusion |
|
8W group: 51 ±10% |
8W group: 51 ± 10 % |
||||||
|
Maxime Tremblay-Gravel et al. |
Cohort (R) |
76 (BRC group: 8, Non-BRC group: 68) |
Death: BRC group: 1
|
BRC group: 23 ± 10 %
|
BRC group: 55 ± 12 %
|
Midterm follow-up: 6 months Long term follow-up: last available follow-up |
None reported |
|
Death: Non-BRC group: 2 |
Non-BRC group: 30 ± 12 % |
Non-BRC group: 45 ± 13 % |
The findings of the study by Sliwa K et al. [27] were supported by a cohort study conducted by Haghikia et al. [28] which had a study population of 115 and a baseline LVEF of 27 ± 9 %. The majority (n=64) of them received a combination of conventional therapy for heart failure and bromocriptine. Among those who were available for the follow-up (n=96), an overwhelming majority (92 %) of the patients in the bromocriptine group showed partial or complete recovery while only 72 % of the patients in the non-bromocriptine group showed recovery. The characteristics and findings of this study are summarized in Table 1. One of the limitations of this study is that the data analyzed did not have proper quality control. Another limitation is that 16.5% of the study population was lost to follow-up. In addition, it was not a randomized placebo-controlled clinical trial [28].
Echoing the findings of the studies conducted by Sliwa K et al. [27] and Haghikia et al. [28], a Randomized Clinical Trial (RCT) of 96 women of African ancestry in Burkina Faso demonstrated better clinical and echocardiographic outcomes in PPCM patients receiving bromocriptine therapy. The baseline mean LVEF was 37.2 ± 6.6 % in patients (n=48) treated with conventional heart failure therapy and bromocriptine, and 37.5 ± 4.8 % in patients (n=48) treated with conventional heart failure therapy alone. Within just two weeks, the group receiving bromocriptine showed better improvement in LVEF compared to the group that did not receive it (LVEF = 41.1 ± 2.1 % vs. 39.6 ± 5.9 %, p=0.021). At six months follow-up, cumulative death was found to be lower (16.6%) in the group treated by bromocriptine compared to the group that was not treated by bromocriptine (29.1 %) (p=0.0001). At 2 months follow-up, the group receiving bromocriptine showed significant improvement in the echocardiographic findings. In this follow-up, it was observed that LVEF was 53.9 ± 4.1 % in the group receiving bromocriptine and 45.9 ± 5.9 % in the group not receiving bromocriptine (p= 0.001) [29]. (Table 1)
In addition, interesting findings were noticed in a study conducted in Germany. In this multicenter prospective randomized study, two different bromocriptine regimens were compared in 63 PPCM patients who were randomized into two groups. One group containing 32 patients received a short course of bromocriptine (2.5 mg bromocriptine for one week) and the other group containing 31 patients received a longer course of bromocriptine (five mg bromocriptine for two weeks followed by 2.5 mg for six weeks). Both groups received standard heart failure therapy and anticoagulant. At six months follow up, the LVEF improved in both the groups (from 28 ± 10 % to 49 ± 12 % in the short-course group and from 27 ± 10 % to 51 ± 10 % in the long-course group). However, the difference in delta-LVEF between the short-course (+ 21 ± 11 %) and the long- course group (+ 24 ± 11 %) was not statistically significant (P = 0.381). Besides, more patients in the long-course group (68 %) showed full recovery (LVEF ≥ 50 %) compared to the short-course group (52 %); however, it was also not statistically significant (p=0.283) [30]. (Table 1)
It is important to mention that although there was no placebo control group in this study, a comparison of a subgroup of this the study characterized by a baseline LVEF of less than 30 % with a subgroup of the Investigation in Pregnancy Associated-Cardiomyopathy (IPAC) study with the same cut off value for LVEF demonstrated a greater clinical recovery in the subgroup of this study. It should be noted that the IPAC subgroup did not receive additional bromocriptine treatment [31]. This favors the narrative that bromocriptine provides a better clinical outcome in PPCM]. However, this finding should be interpreted with caution as the IPAC study subgroup had a much higher percentage of patients of African origin (27 % vs. 2 %) for whom the prognosis tends to be worse.
In a retrospective cohort study by Maxime Tremblay-Gravel et al. [31], bromocriptine was found to be independently associated with better recovery of the left ventricular function. However, in this study, the analysis failed to show any significant association of bromocriptine with mortality in PPCM patients. The characteristics and findings of this study are summarized in Table 1. It should be noted that a very small number of patients (eight) that received bromocriptine treatment were included in this study. Moreover, it was a non-randomized retrospective observational study making it susceptible to several confounders and biases including lack of monitoring of dose and compliance of bromocriptine outside of the hospital, and lack of adjustment of the ethnic background in multivariable analyses.
All of the aforementioned studies have elucidated that bromocriptine has a positive impact on LV function recovery. Recently another study showed that bromocriptine treatment along with standard heart failure therapy is associated with a high rate of recovery of right ventricular function. It is worth mentioning that there was no placebo control arm in this study [32].
On the contrary to these findings which provide supportive evidence in favor of bromocriptine use in PPCM, Anne Schjødt Ersbøll et al.
[38] argued that more evidence is required before considering bromocriptine for PPCM treatment based on post hoc analyses of outcome in two subgroups with baseline LVEF < 30 % in a Danish cohort of PPCM patients. One subgroup had 13 women treated with a prolactin inhibitor drug cabergoline for two days and another subgroup comprised of 23 women without any prolactin inhibitor drug. They found no statistically significant difference in the full recovery rate between these two subgroups. However, this study has significant limitations. Cabergoline was used for only two days instead of the one-week regimen used in most of the studies that showed a better outcome in PPCM with bromocriptine use. Moreover, it was a small, retrospective study.
There have been multiple case reports of procoagulant adverse effects (i.e. MI, stroke) due to the use of bromocriptine in the postpartum period. In fact, in 1995, this led to the withdrawal of FDA approval of bromocriptine for suppression of lactation in the postpartum period [16-18]. Due to these reports, there have been questions about the safety of bromocriptine therapy in PPCM.
James D Fett [33] expressed concerns over the fact that in the study conducted by Hilfiker-Kleiner et al. [30], some patients in one of the study groups experienced adverse events due to thrombosis and/embolism despite getting prophylactic anticoagulant. He also implied that the use of bromocriptine might have been responsible for such adverse outcomes by pointing out that no thromboembolism was observed in 99 subjects of the IPAC study (10) who did not receive bromocriptine. However, Hilfiker-Kleiner et al. [34] argued back that a recent study on PPCM [35] showed that 6.8 % of PPCM patients irrespective of treatment with or without bromocriptine had experienced a thromboembolic event. They also highlighted that all the thromboembolic adverse events reported in their study occurred weeks after cessation of bromocriptine treatment, and hence, argued that the thromboembolic events were not related to bromocriptine. According to them, the underlying disease process may have contributed to such adverse events [34].
It is important to note that in other studies [27, 29, 31] where prophylactic anticoagulation was used along with bromocriptine in PPCM patients, the number of prothrombotic adverse effects was unremarkable. Therefore, prophylactic anticoagulation use might be an effective strategy to reduce the risk of procoagulant adverse effects in PPCM patients taking bromocriptine. However, more studies are required before reaching any conclusion regarding this.
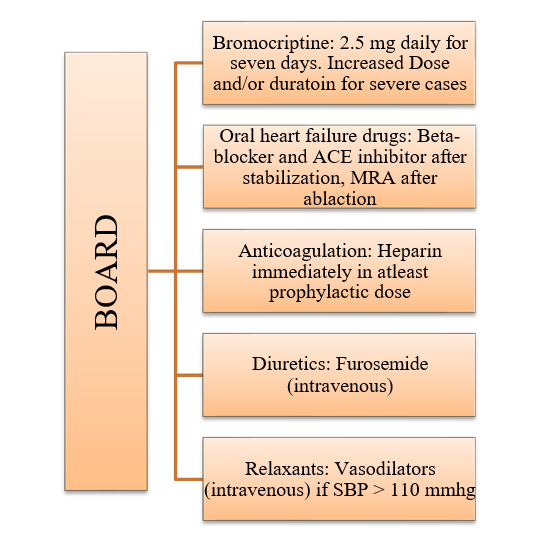
In 2017, Mattia Arrigo et al. [36] proposed the BOARD regimen for the treatment of PPCM. In this regimen, they included bromocriptine as a therapeutic option for all PPCM patients in addition to standard heart failure therapy. Figure 5 summarizes their recommendations. However, they made these recommendations based on the aforementioned study conducted by Hilfiker-Kleiner et al. [30] which was conducted in Germany, and the patient population of this study was overwhelmingly Caucasians. Therefore, more studies are required to assess the effectiveness of the BOARD regimen in treating PPCM patients of diverse ethnic and regional backgrounds.
Figure 5: The ‘BOARD’ regimen. MRA = Mineralocorticoid receptor antagonist, SBP = Systolic blood pressure. (Adapted from ‘Bromocriptine for the treatment of peripartum cardiomyopathy: welcome on BOARD) [58]
Recently, ESC has embraced the BOARD regimen, and now recommends the use of bromocriptine with appropriate anticoagulation in PPCM. As per their guidelines published in 2018, a short-term bromocriptine treatment may be taken into consideration in addition to standard heart failure therapy in uncomplicated PPCM cases. For patients with an ejection fraction of less than 25 % with or without cardiogenic shock, a long-term bromocriptine treatment may be taken into consideration. In addition, they have strongly recommended the use of heparin for anticoagulation, at least in prophylactic dosages, for all patients taking bromocriptine [15].
Future Prospective and Recommendations
Recent studies have shown that adding bromocriptine to the treatment regimen of PPCM provides better clinical and echocardiographic outcomes [39]. However, even in the studies that showed better outcomes with bromocriptine use, not all patients had better outcomes. One of the reasons for this may be the fact that multiple etiological factors are involved in the pathogenesis of PPCM, and bromocriptine treatment helps to counter only one of these factors [31]. For example, as we have mentioned earlier, in a study that demonstrated the role of the absence of PGC-1 alpha and increased sFlt-1 in the pathogenesis of PPCM, treatment of PGC-1 alpha cardiac KO mice were achieved with a combination of bromocriptine and VEGF, not bromocriptine alone. Moreover, as per our discussion earlier, a subset of PPCM with genetic predispositions may be more resistant to various PPCM therapeutic options including bromocriptine. Again, the studies depicting positive outcomes associated with bromocriptine treatment in PPCM were not large- scale international studies with a diverse study population. A randomized control trial (RCT) to detect the effectiveness of bromocriptine in PPCM patients in Canada is already underway and is estimated to be completed by January 1, 2023 (ClinicalTrials.gov, study number: NCT02590601). The results of this study should provide us more evidence to support or disapprove the use of bromocriptine in PPCM. However, we strongly recommend that more RCTs on larger scales with diverse study populations should be done to assess the effectiveness of bromocriptine therapy in various subsets of PPCM patients.
Limitations
Our study is subject to certain limitations. Firstly, since this is a traditional review, it is subject to biases due to the lack of objective and systemic selection criteria. Secondly, as the use of bromocriptine in PPCM is a relatively new concept, enough research has not been done on this topic. For instance, we were able to discuss only 3 RCTs related to this topic in our article.
Conclusion
Recent studies on the pathophysiology of PPCM have revealed that prolactin plays a vital role in the pathogenesis of PPCM. Hence, from a theoretical point of view, it can be deduced that bromocriptine, a prolactin release inhibitor drug, has the potential to become a disease- specific treatment option for PPCM. Our analyses of the available data have also shown that bromocriptine improves clinical and echocardiographic outcomes in PPCM patients. However, despite encouraging results from early observational and interventional studies, the actual efficacy and safety of bromocriptine in real-life PPCM patients is a much-debated topic. Even though it has been recommended to use prophylactic anticoagulant with bromocriptine in PPCM patients, it is vital to conduct more studies that are specifically designed to determine which dosage of bromocriptine and anticoagulant is optimal to get the best possible effectiveness without inducing any major thromboembolic adverse effects. Moreover, recent findings suggesting the association of genetic predisposition with a portion of PPCM cases also warrant further research on the effect of bromocriptine therapy in such cases. It is also important to note that one of the most striking aspects of PPCM is the lack of homogeneity in its regional and ethnic distribution. However, most of the studies to detect the effectiveness of bromocriptine in PPCM have taken place in Europe, and the study population of these studies lacked diversity. Therefore, if more large-scale randomized control trials are conducted in a diverse group of PPCM patients, especially in continents other than Europe, and the results of these trials also show a beneficial effect of bromocriptine in PPCM, it will significantly strengthen the already available pieces of evidence that favor the use of bromocriptine in PPCM. Hence, we conclude from our review that early research does show that bromocriptine has a great prospect to be a disease-specific therapeutic agent for PPCM. But given the heterogeneity in the ethnic and regional distribution and etiological factors of PPCM, large scale international placebo- controlled randomized clinical trials with a diverse study population should be conducted before reaching any absolute conclusion.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Demakis JG, Rahimtoola SH, Sutton GC, Meadows WR, Szanto PB, et al. (1971) Natural course of peripartum cardiomyopathy. Circulation. 44(6): 1053–61.
- Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, et al. (2010) Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 12(8): 767–78.
- Kolte D, Khera S, Aronow WS, Palaniswamy C, Mujib M, et al. (2014) Temporal trends in incidence and outcomes of peripartum cardiomyopathy in the United States: a nationwide population- based study. J Am Heart Assoc. 3(3): e001056.
- Mielniczuk LM, Williams K, Davis DR, Tang ASL, Lemery R, et al. (2006) Frequency of peripartum cardiomyopathy. Am J Cardiol. 97(12): 1765–8.
- Isezuo SA, Abubakar SA (2007) Epidemiologic profile of peripartum cardiomyopathy in a tertiary care hospital. Ethn Dis. 17(2): 228–33.
- Fett JD, Christie LG, Carraway RD, Murphy JG (2005) Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc. 80(12): 1602–6.
- Desai D, Moodley J, Naidoo D (1995) Peripartum cardiomyopathy: experiences at King Edward VIII Hospital, Durban, South Africa and a review of the literature. Trop Doct. 25(3): 118–23.
- Ersbøll AS, Johansen M, Damm P, Rasmussen S, Vejlstrup NG, et al. (2017) Peripartum cardiomyopathy in Denmark: a retrospective, population-based study of incidence, management and outcome. Eur J Heart Fail. 19(12): 1712–1720.
- Gentry MB, Dias JK, Luis A, Patel R, Thornton J, et al. (2010) African American women have a higher risk for developing peripartum cardiomyopathy. J Am Coll Cardiol. 55(7): 654–9.
- McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, et al. (2015) Clinical outcomes for peripartum cardiomyopathy in north America: Results of the IPAC study (Investigations of Pregnancy- Associated Cardiomyopathy). J Am Coll Cardiol. 66(8): 905–14.
- Selle T, Renger I, Labidi S, Bultmann I, Hilfiker-Kleiner D (2009) Reviewing peripartum cardiomyopathy: current state of knowledge. Future Cardiol. 5(2): 175–89.
- van Spaendonck-Zwarts KY, Posafalvi A, van den Berg MP, Hilfiker-Kleiner D, Bollen IAE, et al. (2014) Titin gene mutations are common in families with both peripartum cardiomyopathy and dilated cardiomyopathy. Eur Heart J. 35(32): 2165–73.
- Hilfiker-Kleiner D, Sliwa K, Drexler H (2008) Peripartum cardiomyopathy: recent insights in its pathophysiology. Trends Cardiovasc Med. 18(5): 173–9.
- Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U (2020) Peripartum cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 75(2): 207–221.
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, et al. (2018) 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 39(34): 3165–241.
- Iffy L, Lindenthal J, Mcardle JJ, Ganesh V (1996) Severe cerebral accidents postpartum in patients taking bromocriptine for milk suppression. Isr J Med Sci. 32(5): 309–12.
- Hopp L, Haider B, Iffy L (1996) Myocardial infarction postpartum in patients taking bromocriptine for the prevention of breast engorgement. Int J Cardiol. 57(3): 227–32.
- govinfo [Internet]. Govinfo.gov. [cited 2020 Aug 28].
- Bello NA, Arany Z (2015) Molecular mechanisms of peripartum cardiomyopathy: A vascular/hormonal hypothesis. Trends Cardiovasc Med. 25(6): 499–504.
- Patten IS, Rana S, Shahul S, Rowe GC, Jang C, et al. (2012) Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 485(7398): 333–338.
- Ersbøll AS, Damm P, Gustafsson F, Vejlstrup NG, Johansen M (2017) Peripartum cardiomyopathy: A systematic literature review. Obstet Anesth Dig. 37(3): 119.
- van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ, van der Werf R, Jongbloed JDH, et al. (2010) Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation. 121(20): 2169–75.
- Morales A, Painter T, Li R, Siegfried JD, Li D, et al. (2010) Rare variant mutations in pregnancy-associated or peripartum cardiomyopathy. Circulation. 121(20): 2176–82.
- Liu X, Tang C, Wen G, Zhong C, Yang J, et al. (2018) The mechanism and pathways of dopamine and dopamine agonists in prolactinomas. Front Endocrinol (Lausanne). 9: 768.
- Bromocriptine. [cited 2020 Aug 28].
- Ben-Jonathan N (2001) Dopamine as a Prolactin (PRL) Inhibitor. Endocr Rev. 22(6): 724–63.
- Sliwa K, Blauwet L, Tibazarwa K, Libhaber E, Smedema J-P, et al. (2010) Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. 121(13): 1465–73.
- Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, et al. (2013) Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 108(4): 366.
- Yaméogo NV, Kagambèga LJ, Seghda A, Owona A, Kaboré O, et al. (2017) Bromocriptine in Management of Peripartum Cardiomyopathy: A Randomized Study on 96 Women in Burkina Faso. J Cardiol Clin Res. 5(2): 1098.
- Hilfiker-Kleiner D, Haghikia A, Berliner D, Vogel-Claussen J, Schwab J, et al. (2017) Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomized study. Eur Heart J. 38(35): 2671–2679.
- Tremblay-Gravel M, Marquis-Gravel G, Avram R, Desplantie O, Ducharme A, et al. (2019) The effect of bromocriptine on left ventricular functional recovery in peripartum cardiomyopathy: insights from the BRO-HF retrospective cohort study: Bromocriptine in peripartum cardiomyopathy. ESC Heart Fail. 6(1): 27–36.
- Haghikia A, Schwab J, Vogel-Claussen J, Berliner D, Pfeffer T, et al. (2019) Bromocriptine treatment in patients with peripartum cardiomyopathy and right ventricular dysfunction. Clin Res Cardiol. 108(3): 290–297.
- Fett JD (2018) More study still needed on the use of bromocriptine in the treatment of peripartum cardiomyopathy. Eur Heart J. 39(43): 3904.
- Hilfiker-Kleiner D, Sliwa K, Bauersachs J (2018) Reply to “More study still needed on the use of bromocriptine in the treatment of peripartum cardiomyopathy.” Eur Heart J. 39(43): 3905.
- Sliwa K, Mebazaa A, Hilfiker-Kleiner D, Petrie MC, Maggioni AP, et al. (2017) Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM): EURObservational Research Programme in conjunction with the Heart Failure Association of the European Society of Cardiology Study Group on PPCM. Eur J Heart Fail. 19(9): 1131–1141.
- Arrigo M, Blet A, Mebazaa A (2017) Bromocriptine for the treatment of peripartum cardiomyopathy: welcome on BOARD. Eur Heart J. 38(35): 2680–2682.
- Francis GS, Parks R, Cohn JN (1983) The effects of bromocriptine in patients with congestive heart failure. Am Heart J. 106(1 Pt 1): 100–6.
- Ersbøll AS, Arany Z, Gustafsson F (2018) Bromocriptine for the treatment of peripartum cardiomyopathy: comparison of outcome with a Danish cohort. Eur Heart J. 39(37): 3476–7.
- Croix GS, Ibrahim M, Chaparro S (2018) Use of Bromocriptine in the Management of Peripartum Cardiomyopathy: A Systematic Review and Meta-analysis. Imedpub.com. 2(1): 4.
- Radl DB, Zárate S, Jaita G, Ferraris J, Zaldivar V, et al. (2008) Apoptosis of lactotrophs induced by D2 receptor activation is estrogen dependent. Neuroendocrinology. 88(1): 43–52.
- Kanasaki H, Fukunaga K, Takahashi K, Miyazaki K, Miyamoto E (2000) Involvement of p38 mitogen-activated protein kinase activation in bromocriptine-induced apoptosis in rat pituitary GH3 cells. Biol Reprod. 62(6): 1486–94.
- Yin D, Tamaki N, Kokunai T, Yasuo K, Yonezawa K (1999) Bromocriptine-induced apoptosis in pituitary adenoma cells: relationship to p53 and bcl-2 expression. J Clin Neurosci. 6(4): 326–31.
- Palmeri CM, Petiti JP, Sosa L del V, Gutiérrez S, De Paul AL, et al. (2009) Bromocriptine induces parapoptosis as the main type of cell death responsible for experimental pituitary tumor shrinkage. Toxicol Appl Pharmacol. 240(1): 55–65.
- Geng X, Ma L, Li Z, Li Z, Li J, et al. (2017) Bromocriptine induces autophagy-dependent cell death in pituitary adenomas. World Neurosurg. 100: 407–416.
- Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, et al. (2016) Shared genetic predisposition in peripartum and dilated cardiomyopathies. Obstet Anesth Dig. 36(3): 144–145.