Seda Yılmaz1*, Özcan Çeneli2, Atakan Tekinalp3
1Konya City Hospital, Department of Internal Medicine, Clinic of Hematology, Konya, Turkey
2Necmettin Erbakan University, Meram Faculty of Medicine, Department of Hematology, Konya, Turkey
*Corresponding Author: Seda Yilmaz, Konya City Hospital, Department of Internal Medicine, Clinic of Hematology, Konya, Turkey.
Abstract
Thrombotic microangiopathy demonstrates the general picture characterized by microangiopathic hemolytic anemia, thrombocytopenia, and microvascular thrombus. Pathological processes that cause the development of TMA in different ways have been identified and may have primary and secondary causes. This report presents a case of systemic lupus erythematosus diagnosed during the second thrombotic thrombocytopenic purpura episode at 37—weeks of the pregnancy.
Keywords: Pregnancy; SLE; TTP
Introduction
Thrombotic microangiopathy (TMA) is a process in which platelet and fibrin accumulation in capillary and small vessels, microangiopathic hemolytic anemia, and thrombocytopenia are seen clinically, and organ damage develops related to them. TMAs can be classified under two headings: Thrombotic thrombocytopenic purpura (TTP) is the primary TMA syndrome associated with the hemolytic uremic syndrome, drug-induced, complement-mediated, metabolism, and coagulation. And systemic lupus erythematosus (SLE) is among the secondary causes of TMA, such as malignant hypertension and pregnancy. Typical pathology in TTP is the presence of huge von Willebrand factor (vWF) multimers, platelet- rich thrombi in arterioles and capillaries, and ADAMTS-13 activity level below 10 % is diagnostic. TMA may also develop in systemic autoimmune diseases such as SLE and scleroderma. In this article, a case with a 2. TTP development during pregnancy and a diagnosis of SLE in the same period was presented.
Case
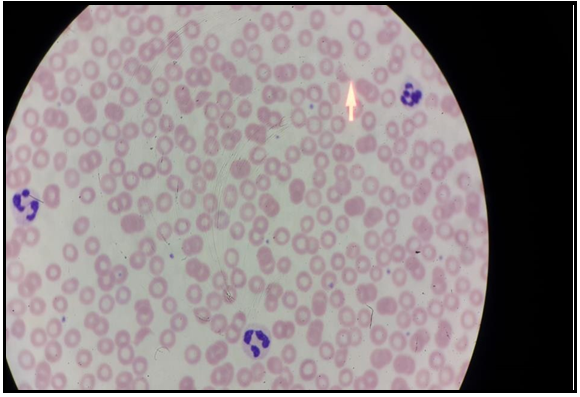
A 34-year-old female patient was referred from the pregnancy clinic for thrombocytopenia, which had been present for about 2 months. Thirty-seven-week pregnant patient received plasmapheresis and methylprednisolone treatment due to the diagnosis of TTP 12 months ago. The patient came to the last hematology clinic follow-up 7 months ago and her platelet value at that time was 197.000 / in. The general condition was good in the physical examination, and both tibia and anterior petechiae were detected. The laboratory values were; WBC 11.400 / µL, hemoglobin 11.2g / dL, platelet 37.000 / µl, and renal and liver functions were normal. LDH was 266 U / L, total bilirubin was 0.42 mg / dL, direct bilirubin was 0.24 mg/dl, haptoglobin was < 0.02 mg/dl, reticulocyte was 6.5 %, direct Coombs (IgG) was +1. In the peripheral smear, fragmentation in erythrocytes and 2-3 platelets were observed in each area (Figure 1).
Figure-1: In the peripheral smear, fragmentation in erythrocytes and 2-3 platelets were observed in each area
Plasmapheresis and methylprednisolone were administered to the patient. Pre-treatment ADAMTS13 activity was < 0.2 %, antigen level was 0.091IU / mL, and inhibitor level was 12.7U / mL. On the 7th day of plasmapheresis, target platelet and LDH values (153,000 / LDL and 150U / mL) were achieved. One week after the end of the plasmapheresis, the expected full-term delivery was performed with a 177.000 / L platelet count, and a healthy baby girl (3160g and 51cm) was born. The patient, who was thought to be a TTP attack, had direct Coombs test positivity, and rheumatologic markers were required to investigate the etiology of the attack and secondary factors that may cause TMA. Anti-nuclear antibody (homogeneous (+++) and cytoplasm-granular (+++)), ENA profile favorable (Ro-52 +; DFS-70 +), anti-dsDNA (+) were found. The patient whose platelet and hemoglobin levels remained stable after delivery was found to have activity of < 0.2 %, antigen level was 0.22IU / mL, and inhibitor level was > 90U / mL when ADAMTS activity was studied again. In light of the present findings, after also discussing with the rheumatologists, she was diagnosed with SLE. The patient received hydroxychloroquine with SLE and TTP diagnosis and followed up in remission.
Discussion
TTP is a rare and life-threatening disease that occurs in about 0.5-2 % of patients with SLE [1,2]. Classical TTP was observed in SLE patients and associated with poor prognosis. ADAMTS activity is variable in SLE-TTP association. In addition to the mechanisms in TTP, free radical-associated proinflammatory process, anti-endothelial antibody, and complement activation have been found responsible. Acute renal injury is more common in secondary TTP patients with SLE [3]. Since there is no specific effective agent, immunosuppressive therapy such as corticosteroid, cyclophosphamide, mycophenolate, azathioprine, and rituximab is administered [4], and plasmapheresis response is variable.
Birth does not cause a decline in the TTP table. In contrast, TTP associated with pregnancy occur more frequently after childbirth.
Therefore, in cases with a diagnosis of TTP or TTP attacks in early pregnancy and pre-term labor should only be considered in cases with high obstetric mortality, such as severe eclampsia. In our case, the TTP attack occurred in the last trimester and was controlled by plasmapheresis. There is no report of TTP transferring from the mother to the baby during the pregnancy. [5,6,7]. Nevertheless, intrauterine death may result from placental infarction due to thrombosis of decidua arterioles [8]. In obstetric controls performed during the treatment, no problem was detected related to the feeding and development of the fetus. The pregnancy continued in the natural flow and resulted in healthy labor.
Conclusion
The relationship between SLE and TTP is rare. The distinction between both diagnoses is essential for treatment and prognosis, and failure to provide appropriate therapy may result in death. A multidisciplinary approach involving hematology and rheumatology is required for early diagnosis and treatment.
References
- Tsai HM (2010) Pathophysiology of thrombotic thrombocytopenic purpura. Int J Hematol. 91(1):1-19.
- Musio F, Bohen EM, Yuan CM, Welch PG (1998) Review of thrombotic thrombocytopenic purpura in the setting of systemic lupus erythematosus. Semin Arthritis Rheum. 28(1): 1-19.
- Letchumanan P, Ng HJ, Lee LH, Thumboo J (2009) A comparison of thrombotic thrombocytopenia purpura in an inception cohort of patients with and without systemic lupus erythematosus. Rheumatology. 48(4): 399–403.
- Hamasaki K, Mimura T, Kanda H, Kubo K, Setoguchi K, et al. (2003) Systemic lupus erythematosus and thrombotic thrombocytopenia purpura: a case report and literature review. Clin Rheumatol. 22(4-5): 355-8.
- Bell WR, Braine HG, Ness PM, Kickler TS (1991) Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med. 325(6): 398-403.
- Egerman RS, Witlin AG, Friedman SA, Sibai BM (1996) Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome in pregnancy: review of 11 cases. Am J Obstet Gynecol. 175(4 Pt 1): 950-6.
- Ezra Y, Rose M, Eldor A (1996) Therapy and prevention of thrombotic thrombocytopenic purpura during pregnancy: a clinical study of 16 pregnancies. Am J Hematol. 51(1): 1-6.
- Wurzei JM (1979) TTP lesions in placenta but not fetus. N Engl J Med. 301: 503-504.