Jussara Soares Fontes1,2, Thalles Trindade de Abreu2, Isabela Gontijo e Couto², Larissa Siqueira Campos¹, Maria Goretti Moreira Guimarães Penido3,4*
1Federal University of São João Del Rei - Faculty of Medicine of Dona Lidu Campus, Divinópolis, Minas Gerais, Brazil
2Pediatric Nephrology Unit - Nephrology Center of Complexo de Saúde São João de Deus, Divinópolis, Minas Gerais, Brazil
3Pediatric Nephrology Unit - Nephrology Center of Santa Casa de Belo Horizonte, Belo Horizonte, Minas Gerais, Brazil
4Federal University of Minas Gerais - Faculty of Medicine - Department of Pediatrics - Pediatric Nephrology Unit, Belo Horizonte, Minas Gerais, Brazil
*Corresponding Author: Maria Goretti Moreira Guimarães Penido, Pediatric Nephrology Unit - Nephrology Center of Santa Casa de Belo Horizonte, Belo Horizonte, Minas Gerais, Brazil. Federal University of Minas Gerais - Faculty of Medicine - Department of Pediatrics - Pediatric Nephrology Unit, Belo Horizonte, Minas Gerais, Brazil. ORCID: 0000-0002-1534-3861
Abstract
Background: Anti-glomerular basement membrane (anti-GBM) disease is a rare entity in the pediatric population characterized by rapidly progressive glomerulonephritis (RPGN), often without pulmonary involvement. It is a double seropositive disease, consisting of anti-GBM antibody plus anti-neutrophil cytoplasmic antibodies. The diagnosis is based on an RPGN clinical presentation combined with serological detection of circulating antibodies and/or direct immunofluorescence of renal biopsy. The recommended treatment is plasmapheresis as soon as possible. Although it has been described as rare in children, its possibility must be considered in the differential diagnosis of acute renal failure and glomerulonephritis.
Case–Diagnosis/Treatment: We report the case of a 13-year-old male patient who presented o the emergency department with fever, fatigue, inappetence, and vomiting. He reported having dark urine with normal urine output. On physical exam, he was pale-looking with tachycardia, tachypnea, and a systolic heart murmur on auscultation. The lungs were normal on auscultation and there were no edema or skin spots. There was significant anemia, Acute kidney injury (AKI), hyperkalemia, depressed iron stores. The assessment of serology showed C-ANCA reagent, P-ANCA not reagent. Hemolysis tests, serum complement, and Anti-GBM antibody were normal. Glomerular hematuria and nephrotic range proteinuria were present. COVID-19 test negative. The diagnosis was confirmed only after the kidney biopsy.
After excluding infections, we started methylprednisolone followed by prednisone, cyclophosphamide orally, and plasmapheresis was initiated. Unfortunately, the patient did not recover renal function and remains on a chronic hemodialysis program.
Conclusion: We do believe that any case of anti-GBM should be described to better understand this rare pediatric disease.
Keywords: Children, anti-GBM, end-stage renal disease, Goodpasture’s disease
Introduction
Anti-glomerular basement membrane (anti-GBM) disease is uncommon in children and is characterized by rapidly progressive glomerulonephritis (RPGN) or pulmonary-renal syndrome with alveolar hemorrhage, known as Goodpasture syndrome [1-3]. It is caused by autoantibodies (classically type G immunoglobulin) directed against the α3 subunit of type IV collagen [2-5]. The autoantibodies target an antigen expressed in the basement membranes of these organs, however, the events that initiate and induce the autoimmune response are not fully understood [2-5]. It is believed that environmental factors, including infection, cigarette smoking, and others can trigger the disease in genetically susceptible individuals [2,6].
Anti-GBM disease occurs in 0.5–1 case per million/year in adult patients [3]. Most affected patients are adolescents and adults of all ages, and the male-to-female ratio varies from 1:1 to 9:1 [7]. About one-quarter of patients will also have antineutrophil cytoplasmic antibodies (ANCA) associated with vasculitis and other kidney diseases such as membranous nephropathy [8,9]. The coexistence of circulating antibodies against GBM and ANCAs (double or dual positivity) in children is extremely rare with only seven patients reported to date [9]. In addition, atypical presentations of anti-GBM disease are increasingly reported [3,10].
The diagnosis is based on an RPGN clinical presentation combined with serological detection of circulating antibodies and/or direct immunofluorescence of renal biopsy specimens. The pathogenic antibodies are usually of the IgG class, with IgG1 and IgG3 subclasses predominating [11]. Cases of IgA- and IgG4-mediated disease have been described, however, they are rare [12]. Conventional direct immunofluorescence techniques will identify all IgG subclasses and may demonstrate the presence of C components, (C3 and C1q), along the GBM. Some patients also demonstrate deposition along tubular basement membranes [13].
As aforementioned, most patients have an RPGN as a consequence of the widespread glomerular crescent formation and 40 %–60 % of them will have concurrent alveolar hemorrhage [3].
According to the 2012 Kidney Disease Improving Global Outcomes guidelines the recommended treatment is plasmapheresis as soon as possible to quickly remove anti-GBM autoantibodies from circulation [5]. Corticosteroids and cyclophosphamide are also recommended to prevent further antibody production and glomerular injury [5]. Studies have related that when this combination of treatments is started early, most patients will have good renal outcomes [2]. De novo anti-GBM disease after transplantation for Alport syndrome is a recognized phenomenon, however, relapse or recurrent disease after kidney transplantation are both uncommon [2].
Authors have suggested that GBM in childhood presents structural differences that change progressively between 3 months and 3 years of age when they will be the same as that of an adult. This fact would contribute to the extremely rare diagnosis of anti-GBM disease in younger children [6,14,15]. Although it has been suggested that anti- GBM disease rarely occurs in children, the possibility of this disease must be considered in the differential diagnosis of acute renal failure and glomerulonephritis.
Considering that this disease is serious, requires immediate treatment and that there is scant data in the literature (no reports in Brazil), we present a case of the anti-GBM disease in a 13-year-old adolescent, who rapidly evolved with definitive loss of renal function.
Case report
A 13-year-old male patient with no report of previous illnesses, hospitalizations, or chronic use of medications presented to the emergency department of a hospital in the southeast region of Minas Gerais/Brazil, for a 15-day history of fever, fatigue, inappetence, and vomiting. He was stable reporting normal urine output and dark urine. A family history of kidney diseases identified a maternal uncle with end-stage chronic kidney disease (ESKD) of unknown etiology who underwent dialysis and kidney transplantation.
On physical examination he had sinus tachycardia (110/min), tachypnea, a systolic heart murmur (grade 2/6) pale, clear lungs to auscultation bilaterally, no edema or skin spots. Body weight 40kg, body height 161cm, BP 140/90mmHg. Laboratory tests showed significant anemia (hemoglobin 3.4g/dL, hematocrit 11.2 %), no changes in the white series or platelets (leukocytes 6,400mm3, platelets 416,000mm3); renal dysfunction (creatinine 5.7mg/dL, urea 159mg/dL, eFGR 11,7 ml/min/1,73m2 body surface), hyperkalemia (potassium 6.4mg/dL), negative hemolysis tests (negative direct coombs, normal haptoglobulin, reticulocytes and bilirubin), depressed iron stores (serum iron 11mg/dL, ferritin 95.5mg/dL, total iron-binding capacity 217, free iron-binding capacity 205, transferrin saturation index 5 %), hyperphosphatemia (6.5mg/dL), metabolic acidosis (venous blood gases pH 7.36, bicarbonate 19.3), serum albumin 3.0g/dL, total cholesterol 112mg/dL, triglycerides 41mg/dL, normal vitamin B12. The assessment of serology showed C-ANCA reagent 1:80, P-ANCA not reagent. Serum complement (C3- 136mg/dL, C4-32mg/dL, CH50-156U/L), FAN, Anti-GBM antibody, rheumatoid factor < 8, protein electrophoresis and viral serology were normal or negative (non-reactive HBsAg, Anti-HBs 14.52; non- reactive HCV). Leishmaniasis negative, Schistosomiasis non- reactive, CMV IgG 71 (+) IgM 0.19 (-), Toxoplasmosis IgG 0.20 (-) IgM 0.13 (-), Epstein Baar IgM 0.11 (-), IgG 46.72 (+), IgE alpha lactalbumin < 0.1, normal hemoglobin electrophoresis. Urine examination showed glomerular hematuria and nephrotic proteinuria (80 RBC/hpf, casts hyaline and granular casts, rare blood casts, codocytes, acanthocytes, spot urine protein to creatinine ratio 2.8). (Table 1 and 2).
Table 1: Laboratory data of the blood samples
|
Test |
Value |
Normal values |
|
Hemoglobin |
3.4 g/dL |
13 – 17 g/dl |
|
Hematocrit |
11.2% |
40-50 % |
|
Leukocytes |
6400 mm3 |
4000 – 10000 mm3 |
|
Platelets |
416,000 mm3 |
150,000 - 400,000 mm3 |
|
Creatinine |
5.7 mg/dL |
0.42 – 0.85 mg/dL |
|
Urea |
159 mg/dL |
19 – 49 mg/dl |
|
GFR |
11,7 ml/min/1,73m2 body surface |
> 60 ml/min/1,73m2 body surface |
|
Potassium |
6.4 mg/dL |
3.5 - 5.5 mg/dL |
|
pH |
7.36 |
7.35 – 7.45 |
|
Bicarbonate |
19.3 mmol/L |
22 – 26 mmol/L |
|
Serum albumin |
3.0 g/dL |
3.5 – 5.2 g/dL |
|
Direct coombs |
Negative |
Negative |
|
Haptoglobulin |
327 mg/dL |
40 – 280 mg/dL |
|
Reticulocytes: Relative Absolute |
0,5 % 14700 /mm³ |
0,5 – 2,17 % 22100 – 96300 mm³ |
|
Bilirubins: Total Direct Indirect |
0,6 mg/dL 0,33 mg/dL 0,27 mg/dL |
< 1,4 mg/dL < 0,4 mg/dL < 1,0 mg/dL |
|
Serum iron |
11 mcg/dL |
65 – 175 mcg/dL |
|
Ferritin |
95.5 ng/dL |
7 – 140 ng/ml |
|
Total iron-binding capacity |
205 mcg/dL |
69 – 240 mcg/dL |
|
Free iron-binding capacity |
217 mcg/dL |
155 – 355 mcg/dL |
|
Transferrin saturation index |
5 % |
20 – 50 % |
|
Phosphorus |
6.5 mg/dL |
2.5 – 5.0 mg/dL |
|
Total cholesterol |
112 mg/dL |
0 – 170 mg/dL |
|
Triglycerides |
41 mg/dL |
0 – 75 mg/dL |
|
Vitamin B12 |
285 pg/ml |
211 – 911 pg/ml |
|
C-ANCA (Methodology: direct immunofluorescence) |
Reagent 1:80 |
NR |
|
P-ANCA |
NR |
NR |
|
C3 |
136 mg/dL |
90 – 170 mg/dL |
|
C4 |
32 mg/dL |
12 – 36 mg/dL |
|
CH50 |
146 u/CAE |
≥ 60 u/CAE |
|
ANA (Methodology: direct immunofluorescence) |
NR |
NR |
|
Anti-GBM antibody |
NR |
NR |
|
Rheumatoid factor |
< 8 ui/ml |
< 8 UI/ml |
|
Protein electrophoresis: Albumina Alfa 1 globulina Alfa 2 globulina Beta 1 globulina Beta 2 globulina Gama globulina Proteínas totais Proteína monoclonal |
2,37 g/dL 0,44 g/dL 0,76 g/dL 0,31 g/dL 0,33 g/dL 0,60 g/dL 4,8 g/dL Absent |
3,5 – 4,85 g/dL 0,22 – 0,43 g/dL 0,55 – 1,08 g/dL 0,32 – 0,54 g/dL 0,24 – 0,54 g/dL 0,74 – 1,75 g/dL 6,5 – 8,2 g/dL Absent |
|
HBsAg |
NR |
NR |
|
Anti-HBs |
14.52 UI/L |
< 10 UI/L |
|
Anti-HBC IgM |
NR |
NR |
|
Anti-HBC IgG |
NR |
NR |
|
HBE-AG |
0,59 |
< 1,0 |
|
HCV |
NR |
NR |
|
Leishmaniasis IgM |
NR |
NR (< 1:80) |
|
Leishmaniasis IgG |
NR |
NR (< 1:80) |
|
Schistosomiasis |
NR |
NR |
|
CMV IgG |
71 AU/ml (+) |
< 6 AU/ml |
|
CMV IgM |
0.19 (-) |
< 0,85 |
|
Toxoplasmosis IgG |
0.20 (-) UI/ml |
< 1,6 UI/ml |
|
Toxoplasmosis IgM |
0.13 (-) |
< 0,5 |
|
Epstein Barr IgM |
0.11 (-) |
|
|
Epstein Barr IgG |
46.72 (+) |
< 0,75 |
|
IgE alpha lactalbumin |
< 0.1 KU/L |
<0,1 KU/L |
|
Hemoglobin electrophoresis: A1 A2 F S C Others |
96,9 % 2,7 % 0,4 % 0 % 0 % 0 %) |
> 95 % 1,5 – 3,7 % < 2% Absent Absent Absent |
|
TSH |
3,97 microUI/mL |
0,38 – 5,33 microUI/mL |
|
T4 Livre |
1,29 ng/dL |
0,89 – 1,76 ng/dL |
|
Covid19 |
Negative |
Negative |
NR: non-reactive, GFR: Glomerular filtration rate, ANCA: Antineutrophil Cytoplasmic Antibodies, ANA: Anti-nuclear antibodies, Anti-GBM: Anti-glomerular basement membrane, CMV: Cytomegalovirus.
Table 2: Laboratory data of the urine samples
|
Test |
Value |
Normal values |
|
Protein |
++ |
Negative |
|
Glucose |
Negative |
Negative |
|
Hemoglobin |
+++ |
Negative |
|
Nitrite |
Negative |
Negative |
|
Leukocytes |
Negative |
Negative |
|
Sedimentoscopia: Red blood cells Leukocytes Hyaline Casts Granular Casts Fatty Casts Blood casts |
80/hpf 5/ hpf Presents Presents Presents Presents |
0-3 0-5 Absent Absent Absent Absent |
|
Dysmorphism of urinary RBC |
Presence of codocytes and acanthocytes |
Negative |
|
P/C ratio |
2,800 mg/g |
< 150 mg/g |
RBC: red blood cells, P/C ratio: Spot urine protein to creatinine ratio.
The patient had a negative test for COVID-19 and he denied gastrointestinal bleeding, hemoptysis, or recent skin and throat infections. He received several red blood cell transfusions due to constant drops in hemoglobin, intravenous hydrocortisone and there was clinical control of hyperkalemia and acidosis.
Renal ultrasound showed kidneys in the right place with preserved dimensions, parenchyma with normal thickness, and diffusely increased echogenicity. Echocardiogram with preserved biventricular systolic function (LV ejection fraction 68%-Simpson). Negative myelogram for leishmaniasis, hemophagocytic syndromes, or other hematologic malignancies. Bone marrow with hyperplasia of the erythrocyte series without maturation of erythroid precursors. Kidney biopsy confirmed rapidly progressive crescentic glomerulonephritis in a proliferative/sclerosing phase related to the anti-GBM disease.
After excluding infections, we started immunosuppression with methylprednisolone 1.0g intravenously for 3 days, followed by prednisone 40mg/day and cyclophosphamide 50mg/day orally. Plasmapheresis was initiated (6 sessions) and corticosteroid therapy was maintained. Unfortunately, the patient did not recover renal function and required dialysis therapy.
Discussion
Few pediatric cases of the anti-GBM disease are reported. According to the literature, the majority of pediatric cases present as an RPGN. However, some cases can present with pulmonary manifestations called Goodpasture syndrome [2,3,9,10]. Here we described the case of a 13-year-old boy with the diagnosis of anti-GBM disease based on kidney biopsy and on the clinical presentation of RPGN, without pulmonary involvement. The initial signs and symptoms in our patient were fever, fatigue, inappetence, and vomiting, quite similar to those described by Bogdanović et al. [9]. According to McAdoo et al., 80 %–90 % of patients will present features of RPGN, 40 %-60 % will also have lung hemorrhage, and a small minority may present with isolated pulmonary disease [2]. It has been recognized as an atypical presentation of anti-GBM disease, often with less renal impairment, although it is unclear whether these represent distinct clinical subphenotypes or a spectrum of disease severity [2]. Studies on the adult populations with anti-GBM reported equal sex prevalence, however, it is not defined in the pediatric group [3].
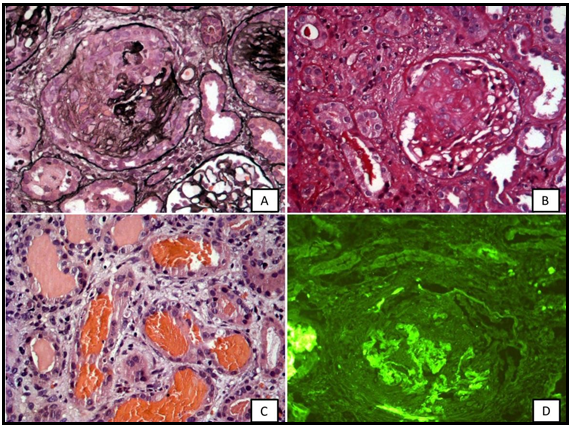
Our patient had hematuria, nephrotic range proteinuria, severe anemia (hemoglobin=3.4g/dL) with decreased iron stores, AKI (creatinine=5.7mg/dL) with hyperkalemia, and hyperphosphatemia, in need of dialysis treatment. Initially, he received several red blood cell transfusions, intravenous hydrocortisone, and furosemide. The biopsy was performed with a sample of 29 glomeruli: four with active cellular crescents, two with fibro cellular crescents, 21 (72 %) with fibrous crescents, and mild/moderate fibrosis (30/40 %). Immunofluorescence reveals strong linear pattern positivity for IgG (+++/3+) in capillary loops along the basal membrane. Hematuria, nephrotic proteinuria, renal dysfunction, and its repercussion characterize an RPGN requiring kidney biopsy. Williamson et al. reported four pediatric anti-GBM cases and all had serum creatinine > 5.7mg/dL and glomerular crescents >85 % in most of them [6]. Dorval et al. identified in the literature 27 pediatric cases of anti-GBM and at diagnosis, the mean serum creatinine was 6.26±4mg/dL (n=25), and 15 children were dialyzed. Five patients died within the first two months (survival rate=81.5 %), 12 (44.5 %) had an independent renal function after a mean follow-up of two years, among dialyzed patients only three recovered renal function, and four (14.8 %) patients underwent kidney transplantation [3]. The authors also related that serum creatinine > 5.7mg/dL at diagnosis and crescentic glomeruli > 85 % are poor prognosis indicators in children [3]. However, Nagano et al. observed the anti-GBM disease in an 8-year-old girl with normal renal function (creatinine=0.4mg/dL) who had participated in a school urine screening program. The renal biopsy with 12 glomeruli showed no crescent formation. The diagnosis was confirmed with positive and high anti-GBM antibodies [15].
Williamson et al. described an 8-year-old female patient with no demonstrable circulating anti-GBM antibodies, despite prominent linear IgG staining in the glomerular and alveolar basement membrane, in IF [6]. About 2–3 % of patients with the anti-GBM disease may have circulating antibody levels that are undetectable by standard assays, but detectable by a specialized biosensor [6]. In these cases, the diagnosis could be made based on biopsy findings. Exactly as mentioned in the case above, the assessment of the anti-GBM antibody in our patient resulted in negative and the IF revealed strong linear pattern positivity for IgG in capillary loops along the basal membrane (Figure 1).
A pathogenic role for anti-GBM antibodies is supported by clinical observations and antibody titer, subclass, and avidity for the basement membrane have been correlated with disease outcomes [2]. It is known that plasma exchange is associated with better outcomes. The immunosuppression in our patient was done with intravenous methylprednisolone, followed by prednisone and cyclophosphamide orally. Concomitantly, plasmapheresis was initiated. This treatment is in accordance with the 2012 Kidney Disease Improving Global Outcomes guidelines recommendations [5]. The treatment for anti-GBM in the pediatric population is extrapolated from adult guidelines. Without the promptness of immunosuppressive therapy, the outcome is poor and natural evolution leads to ESKD and/or death [3,6,10,15].
In conclusion, we described a case of a young adolescent with an acute and poor outcome in which the anti-GBM antibodies were negative. The diagnosis was confirmed only after the kidney biopsy, although the clinical onset was an RPGN. He was treated promptly according to recommended guidelines. However, he did not regain his renal function completely and required chronic hemodialysis. We do believe that any case of anti-GBM should be described in order to better understand this rare disease.
Figure 1: Kidney biopsy. A) Jone´s stain (40X) Glomeruli injured showing rupture of the glomerular basement membrane (GBM) and proliferation of the Bowman´s capsular parietal cells with fibrocelullar crescents. B) PAS stain (40X) presenting a glomeruli tuffs evolved by fibrous crescent. C) H&E (40X) Several tubules with red cells casts. D) Immunofluorescence (40X Leica 2500 Fluorescent Microscopy 100Watts Mercury Lamp) Linear IgG stain along the GBM.
Source: Authors' personal archive.
Statements and Declarations: The authors declare no conflicts of interest between the investigators and the patients, and other institutions.
Funding: None.
Indication of authors' contribution:
Jussara Soares Fontes, Thalles Trindade de Abreu, Larissa Siqueira Campos, Isabela Gontijo e Couto and Maria Goretti Moreira Guimarães Penido were responsible for the research idea, study design, data acquisition, supervision or mentorship and article writing.
References
- Williamson SR, Phillips CL, Andreoli SP, Nailescu C (2011) A 25-year experience with pediatric anti-glomerular basement membrane disease. Pediatr Nephrol. 26: 85–91.
- McAdoo SP, Pusey CD (2017) Anti-Glomerular Basement Membrane Disease. Clin J Am Soc Nephrol. 12(7): 1162–1172.
- Dorval G, Lion M, Guérin S, Krid S, Galmiche-Rolland L, et al. (2017) Immunoadsorption in Anti-GBM Glomerulonephritis: Case Report in a Child and Literature Review. Pediatrics. 140(5): e20161733.
- Ohlsson S, Herlitz H, Lundberg S, Selga D, Mölne J, et al. (2014) Circulating anti-glomerular basement membrane antibodies with predominance of subclass IgG4 and false-negative immunoassay test results in anti-glomerular basement membrane disease. Am J Kidney Dis. 63(2): 289–293.
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, et al. (2013) 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 65(1): 1-11.
- Williamson SR, Phillips CL, Andreoli SP, Nailescu C (2011) A 25-year experience with pediatric anti-glomerular basement membrane disease. Pediatr Nephrol. 26(1): 85–91.
- Shah MK, Hugghins SY (2002) Characteristics and outcomes of patients with Goodpasture’s syndrome. South Med J. 95(12): 1411–1418.
- Helander L, Hanna M, Annen K (2021) Pediatric double positive anti-glomerular basement membrane antibody and anti-neutrophil cytoplasmic antibody glomerulonephritis - A case report with review of literature. J Clin Apheresis. 36(3): 505-510.
- Bogdanović R, Minić P, Marković-Lipkovski J, Stajić N, Savić N, et al. (2013) Pulmonary renal syndrome in a child with coexistence of anti-neutrophil cytoplasmic antibodies and anti- glomerular basement membrane disease: case report and literature review. BMC Nephrol. 14: 66.
- Mannemuddhu SS, Clapp W, Modica R, Elder ME, Upadhyay K (2019) End-stage renal disease secondary to antiglomerular basement membrane disease in a child with common variable immunodeficiency. Clin Nephrol Case Stud. 7: 1-6.
- Zhao J, Yan Y, Cui Z, Yang R, Zhao MH (2009) The immunoglobulin G subclass distribution of anti-GBM autoantibodies against rHalpha3(IV)NC1 is associated with disease severity. Hum Immunol. 70(6): 425–429.
- Moulis G, Huart A, Guitard J, Fortenfant F, Chauveau D (2012) IgA mediated anti-glomerular basement membrane disease: An uncommon mechanism of Goodpasture’s syndrome. Clin Kidney J. 5(6): 545–548.
- Fischer EG, Lager DJ (2006) Anti-glomerular basement membrane glomerulonephritis: A morphologic study of 80 cases. Am J Clin Pathol. 125(3): 445–450.
- Anand SK, Landing BH, Heuser ET, Olson DL, Grushkin CM, et al. (1978) Changes in glomerular basement membrane antigen(s) with age. J Pediatr 92(6): 952–953.
- Nagano C, Goto Y, Kasahara K, Kuroyanagi Y (2015) Case report: anti-glomerular basement membrane antibody disease with normal renal function. BMC Nephrol. 16: 185.